Detection and quantification of a wide range of fMRI temporal responses using a physiologically-motivated basis set
- PMID: 14601143
- PMCID: PMC1866291
- DOI: 10.1002/hbm.10136
Detection and quantification of a wide range of fMRI temporal responses using a physiologically-motivated basis set
Abstract
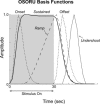
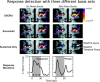
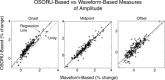
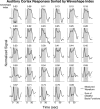
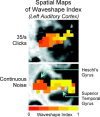
The temporal dynamics of fMRI responses can span a broad range, indicating a rich underlying physiology, but also posing a significant challenge for detection. For instance, in human auditory cortex, prolonged sound stimuli ( approximately 30 sec) can evoke responses ranging from sustained to highly phasic (i.e., characterized by prominent peaks just after sound onset and offset). In the present study, we developed a method capable of detecting a wide variety of responses, while simultaneously extracting information about individual response components, which may have different neurophysiological underpinnings. Specifically, we implemented the general linear model using a novel set of basis functions chosen to reflect temporal features of cortical fMRI responses. This physiologically-motivated basis set (the "OSORU" basis set) was tested against (1) the commonly employed "sustained-only" basis "set" (i.e., a single smoothed "boxcar" function), and (2) a sinusoidal basis set, which is capable of detecting a broad range of responses, but lacks a direct relationship to individual response components. On data that included many different temporal responses, the OSORU basis set performed far better overall than the sustained-only set, and as well or better than the sinusoidal basis set. The OSORU basis set also proved effective in exploring brain physiology. As an example, we demonstrate that the OSORU basis functions can be used to spatially map the relative amount of transient vs. sustained activity within auditory cortex. The OSORU basis set provides a powerful means for response detection and quantification that should be broadly applicable to any brain system and to both human and non-human species.
Copyright 2003 Wiley-Liss, Inc.
Figures
References
-
- Andersen AH, Gash DM, Avison MJ (1999): Principal component analysis of the dynamic response measured by fMRI: a generalized linear systems framework. Magn Reson Imag 17: 795–815. - PubMed
-
- Andrade A, Paradis AL, Rouquette S, Poline JB (1999): Ambiguous results in functional neuroimaging data analysis due to covariate correlation. Neuroimage 10: 483–486. - PubMed
-
- Ardekani BA, Kanno I (1998): Statistical methods for detecting activated regions in functional MRI of the brain. Magn Reson Imag 16: 1217–25. - PubMed
-
- Ardekani BA, Kershaw J, Kashikura K, Kanno I (1999): Activation detection in functional MRI using subspace modeling and maximum likelihood estimation. IEEE Trans Med Imag 18: 101–114. - PubMed
-
- Bandettini PA, Kwong KK, Davis TL, Tootell RBH, Wong EC, Fox PT, Belliveau JW, Weisskoff RM, Rosen BR (1997): Characterization of cerebral blood oxygenation and flow changes during prolonged brain activation. Hum Brain Mapp 5: 93–109. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

