Aerobic denitrification of Pseudomonas aeruginosa monitored by online NAD(P)H fluorescence
- PMID: 14602632
- PMCID: PMC262322
- DOI: 10.1128/AEM.69.11.6715-6722.2003
Aerobic denitrification of Pseudomonas aeruginosa monitored by online NAD(P)H fluorescence
Abstract
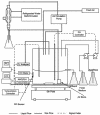
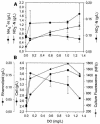
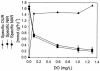
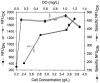
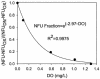
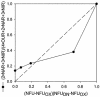
Continuous cultures of Pseudomonas aeruginosa (ATCC 9027) maintained at different dissolved oxygen concentrations (DO) were studied for the effects of DO on various culture properties, especially aerobic respiration and denitrification. The DO was varied from 0 mg/liter (completely anoxic conditions) to 1.3 mg/liter and measured with optical sensors that could accurately determine very low DO based on oxygen-quenched luminescence. The strain was found to perform aerobic denitrification; while the specific rate decreased with increasing DO, denitrification persisted at approximately 1/8 of the maximum rate (1.7 mmol/g of cells/h) even at relatively high DO (1 to 1.3 mg/liter). In the presence of nitrate, the culture's Monod half-rate saturation constant for O(2) was very small, <0.1 mg/liter. Aerobic denitrification appeared to function as an electron-accepting mechanism supplementary to or competitive with aerobic respiration. The shift of the culture's respiratory mechanism was also clearly detected with a fluorometer targeting intracellular NAD(P)H, i.e., the reduced forms of the NAD(P) coenzymes. Comparatively, the NAD(P)H fluorescence under the anoxic, denitrifying conditions (NFU(DN)) was highest, that under fully aerobic conditions (NFU(OX)) was lowest, and that under conditions in which both denitrification and aerobic respiration occurred (NFU) was intermediate. Representing a quantitative measure of the culture's "fractional approach" to the fully denitrifying state, the normalized fraction (NFU - NFU(OX))/(NFU(DN) - NFU(OX)) was correlated with DO and the calculated fraction of electrons accepted by denitrification. The NFU fraction decreased with increasing DO, following an empirical exponential relationship. The fraction of denitrification-accepted electrons increased with the NFU fraction: the increase was gradual and approximately linear at DO of >/==" BORDER="0">0.1 mg/liter but much sharper at lower DO. Online NAD(P)H fluorescence was demonstrated as a feasible technique for effective monitoring and quantitative description of the microaerobic state of microorganisms.
Figures



References
-
- Arino, S., R. Marchal, and J. P. Vandecasteele. 1998. Involvement of a rhamnolipid-producing strain of Pseudomonas aeruginosa in the degradation of polycyclic aromatic hydrocarbons by a bacterial community. J. Appl. Microbiol. 84:769-776. - PubMed
-
- Boulding, J. R. (ed.). 1996. EPA environmental engineering source book. Ann Arbor Press, Chelsea, Mich.
-
- Campos de Alaniz, J. D., and A. A. Solari. 1973. Use of hydrocarbons as the only source of carbon in Pseudomonas aeruginosa. Bioquimica Clinica 7:37-45.
-
- Chayabutra, C., J. Wu, and L.-K. Ju. 2001. Rhamnolipid production by Pseudomonas aeruginosa under denitrification: effects of limiting nutrients and carbon substrates. Biotechnol. Bioeng. 72:25-33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

