The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids?
- PMID: 14602815
- PMCID: PMC6740859
- DOI: 10.1523/JNEUROSCI.23-31-10013.2003
The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids?
Abstract
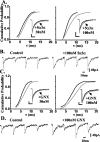
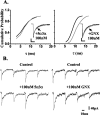
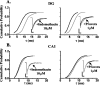
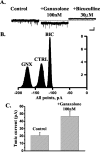
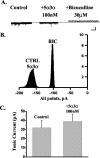
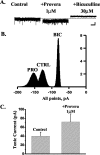
Neurosteroids typified by 5alpha-pregnan-3alpha-ol-20-one (5alpha3alpha) have emerged as the most potent endogenous positive modulators of the GABAA receptor, the principal mediator of fast inhibitory transmission within the CNS. Neurosteroids can be synthesized de novo in the brain in levels sufficient to modulate GABA(A) receptor function and, thus, might play an important physiological-pathophysiological role. Indirect support for this proposal comes from the observation that neurosteroid action is region and neuron selective. However, the mechanism(s) that imparts specificity of action remains primarily elusive. Although neurosteroids are relatively promiscuous toward different GABA(A) receptor isoforms, the contribution of local neurosteroid metabolism has been relatively unexplored. Here, we investigate the role of neurosteroid metabolism by using electrophysiological techniques to compare the actions of 5alpha3alpha and its metabolically stable synthetic analog ganaxolone on inhibitory neurotransmission in CA1 and dentate gyrus neurons. Furthermore, we evaluate the contribution of a key enzyme in neurosteroid metabolism [i.e., 3alpha-hydroxysteroidoxidoreductase (3alpha-HSOR)] to the inactivation of endogenous, or exogenously applied 5alpha3alpha. We show that low concentrations of ganaxolone, but not of 5alpha3alpha, enhance inhibitory transmission in dentate gyrus, whereas both steroids are similarly effective in CA1 neurons. Furthermore, inhibition of 3alpha-HSOR by the contraceptive agent Provera results in enhanced synaptic and extrasynaptic GABA(A) receptor-mediated inhibition in the dentate gyrus but not in the CA1 region. Collectively, these findings advocate a crucial role for local steroid metabolism in shaping GABA(A) receptor-mediated inhibition in a regionally dependent manner and suggest a novel action by the contraceptive agent on inhibitory centers in the CNS.
Figures
References
-
- Belelli D, Lan NC, Gee KW ( 1990) Anticonvulsant steroids and the GABA/benzodiazepine receptor-chloride ionophore complex. Neurosci Biobehav Rev 14: 315-322. - PubMed
-
- Belelli D, Lambert JJ, Peters JA, Gee KW, Lan NC ( 1996b) Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology 35: 1223-1231. - PubMed
-
- Belelli D, Casula A, Ling A, Lambert JJ ( 2002) The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43: 651-661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
