Two modes of vesicle recycling in the rat calyx of Held
- PMID: 14602833
- PMCID: PMC6740849
- DOI: 10.1523/JNEUROSCI.23-31-10164.2003
Two modes of vesicle recycling in the rat calyx of Held
Abstract
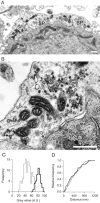
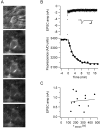
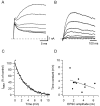
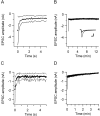
Vesicle recycling was studied in the rat calyx of Held, a giant brainstem terminal involved in sound localization. Stimulation of brain slices containing the calyx-type synapse with a high extracellular potassium ion concentration in the presence of horseradish peroxidase resulted within several minutes in a reduction of the number of neurotransmitter vesicles and in the appearance of labeled endosome-like structures. After returning to normal solution, the endosome-like structures disappeared over a period of several minutes, whereas simultaneously the number of labeled vesicles increased. A comparison with afferent stimulation suggested that the endosome-like structures normally do not participate in the vesicle cycle. Afferent stimulation at 5 Hz resulted in sustained synaptic transmission, without vesicle depletion but with an estimated endocytotic activity of <0.2 synaptic vesicles per active zone per second. At 20 Hz, the presynaptic action potentials generally failed during prolonged stimulation. In identified synapses, the number of vesicles labeled by photoconversion after stimulation at 5 Hz in the presence of the styryl dye RH414 was much lower than the number of vesicles that were released, as determined by measuring EPSCs. No more than approximately 5% of the vesicles were labeled after 20 min stimulation at 5 Hz, whereas this stimulation protocol was sufficient to largely destain a terminal after previous loading. The results support a scheme for recycling in which two different modes coexist. At physiological demands, a pool of approximately 5% of all vesicles provides sufficient vesicles for release. During intense stimulation, such as occurs in the presence of high extracellular K+, the synapse resorts to bulk endocytosis, a very slow mode of recycling.
Figures



References
-
- Aravanis AM, Pyle JL, Tsien RW ( 2003) Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423: 643-647. - PubMed
-
- Borst JGG, Sakmann B ( 1996) Calcium influx and transmitter release in a fast CNS synapse. Nature 383: 431-434. - PubMed
-
- Forsythe ID, Barnes-Davies M ( 1993) The binaural auditory pathway: excitatory amino acid receptors mediate dual time course excitatory postsynaptic currents in the rat medial nucleus of the trapezoid body. Proc Roy Soc Lond B Biol Sci 251: 151-157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
