DNA-protein cross-linking by trans-[PtCl(2)(E-iminoether)(2)]. A concept for activation of the trans geometry in platinum antitumor complexes
- PMID: 14602903
- PMCID: PMC275558
- DOI: 10.1093/nar/gkg863
DNA-protein cross-linking by trans-[PtCl(2)(E-iminoether)(2)]. A concept for activation of the trans geometry in platinum antitumor complexes
Abstract
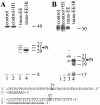
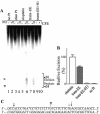
The structure-pharmacological activity relationships generally accepted for antitumor platinum compounds stressed the necessity for the cis-[PtX(2)(amine)(2)] structure while the trans-[PtX(2)(amine)(2)] structure was considered inactive. However, more recently, several trans-platinum complexes have been identified which are potently toxic, antitumor-active and demonstrate activity distinct from that of conventional cisplatin (cis-[PtCl(2)(NH(3))(2)]). We have shown in the previous report that the replacement of ammine ligands by iminoether in transplatin (trans-[PtCl(2)(NH(3))(2)]) results in a marked enhancement of its cytotoxicity so that it is more cytotoxic than its cis congener and exhibits significant antitumor activity, including activity in cisplatin-resistant tumor cells. In addition, we have also shown previously that this new trans compound (trans-[PtCl(2)(E-iminoether)(2)]) forms mainly monofunctional adducts at guanine residues on DNA, which is generally accepted to be the cellular target of platinum drugs. In order to shed light on the mechanism underlying the antitumor activity of trans-[PtCl(2)(E-iminoether)(2)] we examined oligodeoxyribonucleotide duplexes containing a single, site-specific, monofunctional adduct of this transplatin analog by the methods of molecular biophysics. The results indicate that major monofunctional adducts of trans-[PtCl(2)(E-iminoether)(2)] locally distort DNA, bend the DNA axis by 21 degrees toward the minor groove, are not recognized by HMGB1 proteins and are readily removed from DNA by nucleotide excision repair (NER). In addition, the monofunctional adducts of trans-[PtCl(2)(E-iminoether)(2)] readily cross-link proteins, which markedly enhances the efficiency of this adduct to terminate DNA polymerization by DNA polymerases in vitro and to inhibit removal of this adduct from DNA by NER. It is suggested that DNA-protein ternary cross-links produced by trans-[PtCl(2)(E-iminoether)(2)] could persist considerably longer than the non-cross-linked monofunctional adducts, which would potentiate toxicity of this antitumor platinum compound toward tumor cells sensitive to this drug. Thus, trans-[PtCl(2)(E-iminoether)(2)] represents a quite new class of platinum antitumor drugs in which activation of trans geometry is associated with an increased efficiency to form DNA-protein ternary cross-links thereby acting by a different mechanism from 'classical' cisplatin and its analogs.
Figures


References
-
- O'Dwyer P.J., Stevenson,J.P. and Johnson,S.W. (1999) Clinical status of cisplatin, carboplatin and other platinum-based antitumor drugs. In Lippert,B. (ed.), Cisplatin. Chemistry and Biochemistry of a Leading Anticancer Drug. VHCA, WILEY-VCH, Zürich, Weinheim, pp. 31–72.
-
- Farrell N., Kelland,L.R., Roberts,J.D. and Van Beusichem,M. (1992) Activation of the trans geometry in platinum antitumor complexes: a survey of the cytotoxicity of trans complexes containing planar ligands in murine-L1210 and human tumor panels and studies on their mechanism of action. Cancer Res., 52, 5065–5072. - PubMed
-
- Wong E. and Giandomenico,C.M. (1999) Current status of platinum-based antitumor drugs. Chem. Rev., 99, 2451–2466. - PubMed
-
- Judson I. and Kelland,L.R. (2000) New developments and approaches in the platinum arena. Drugs, 59, 29–36. - PubMed
-
- Brabec V. (2002) DNA modifications by antitumor platinum and ruthenium compounds: their recognition and repair. Prog. Nucleic Acids Res. Mol. Biol., 71, 1–68. - PubMed

