Solution structures of stem-loop RNAs that bind to the two N-terminal RNA-binding domains of nucleolin
- PMID: 14602904
- PMCID: PMC275560
- DOI: 10.1093/nar/gkg866
Solution structures of stem-loop RNAs that bind to the two N-terminal RNA-binding domains of nucleolin
Abstract
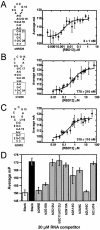
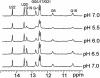
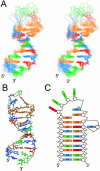
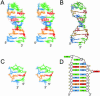
Nucleolin, a multi-domain protein involved in ribosome biogenesis, has been shown to bind the consensus sequence (U/G)CCCG(A/G) in the context of a hairpin loop structure (nucleolin recognition element; NRE). Previous studies have shown that the first two RNA-binding domains in nucleolin (RBD12) are responsible for the interaction with the in vitro selected NRE (sNRE). We have previously reported the structures of nucleolin RBD12, sNRE and nucleolin RBD12-sNRE complex. A comparison of free and bound sNRE shows that the NRE loop becomes structured upon binding. From this observation, we hypothesized that the disordered hairpin loop of sNRE facilitates conformational rearrangements when the protein binds. Here, we show that nucleolin RBD12 is also sufficient for sequence- specific binding of two NRE sequences found in pre-rRNA, b1NRE and b2NRE. Structural investigations of the free NREs using NMR spectroscopy show that the b1NRE loop is conformationally heterogeneous, while the b2NRE loop is structured. The b2NRE forms a hairpin capped by a YNMG-like tetraloop. Comparison of the chemical shifts of sNRE and b2NRE in complex with nucleolin RBD12 suggests that the NRE consensus nucleotides adopt a similar conformation. These results show that a disordered NRE consensus sequence is not a prerequisite for nucleolin RBD12 binding.
Figures


References
-
- Srivastava M. and Pollard,H.B. (1999) Molecular dissection of nucleolin’s role in growth and cell proliferation: new insights. FASEB J., 13, 1911–1922. - PubMed
-
- Ginisty H., Sicard,H., Roger,B. and Bouvet,P. (1999) Structure and function of nucleolin. J. Cell Sci., 112, 761–772. - PubMed
-
- Roger B., Moisand,A., Amalric,F. and Bouvet,P. (2002) Repression of RNA polymerase I transcription by nucleolin is independent of the RNA sequence that is transcribed. J. Biol. Chem., 277, 10209–10219. - PubMed
-
- Bouvet P., Diaz,J.-J., Kindbeiter,K., Madjar,J.-J. and Amalric,F. (1998) Nucleolin interacts with several ribosomal proteins through its RGG domain. J. Biol. Chem., 273, 19025–19029. - PubMed
-
- Ghisolfi-Nieto L., Joseph,G., Puvion-Dutilleul,F., Amaric,F. and Bouvet,P. (1996) Nucleolin is a sequence specific RNA-binding protein—characterization of targets on pre-ribosomal RNA. J. Mol. Biol., 260, 34–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

