Performance of the focus and Kalon enzyme-linked immunosorbent assays for antibodies to herpes simplex virus type 2 glycoprotein G in culture-documented cases of genital herpes
- PMID: 14605166
- PMCID: PMC262548
- DOI: 10.1128/JCM.41.11.5212-5214.2003
Performance of the focus and Kalon enzyme-linked immunosorbent assays for antibodies to herpes simplex virus type 2 glycoprotein G in culture-documented cases of genital herpes
Abstract
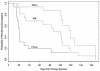
Glycoprotein G-based herpes simplex virus type 2 (HSV-2) enzyme-linked immunosorbent assays from Focus and Kalon were performed with specimens from 118 patients with culture-documented genital herpes episodes, and their results were compared. Sensitivity was 52% by Kalon and 86% by Focus for first HSV-2 episodes and 100% (for each of the two tests) for recurrent HSV-2. Median times to seroconversion were 120 days by the Kalon assay, 21 days by the Focus assay, and 68 days by Western blotting assay. Values for specificity were 100% (Kalon) and 93% (Focus).
Figures

Similar articles
-
Performance of HerpeSelect and Kalon assays in detection of antibodies to herpes simplex virus type 2.J Clin Microbiol. 2008 Jun;46(6):1914-8. doi: 10.1128/JCM.02332-07. Epub 2008 Apr 2. J Clin Microbiol. 2008. PMID: 18385443 Free PMC article. Clinical Trial.
-
False-negative type-specific glycoprotein G antibody responses in STI clinic patients with recurrent HSV-1 or HSV-2 DNA positive genital herpes, The Netherlands.Sex Transm Infect. 2016 Jun;92(4):257-60. doi: 10.1136/sextrans-2015-052213. Epub 2016 Jan 11. Sex Transm Infect. 2016. PMID: 26755775
-
Herpes simplex virus type 2 antibody detection performance in Kisumu, Kenya, using the Herpeselect ELISA, Kalon ELISA, Western blot and inhibition testing.Sex Transm Infect. 2009 Apr;85(2):92-6. doi: 10.1136/sti.2008.031815. Epub 2008 Oct 27. Sex Transm Infect. 2009. PMID: 18955387 Free PMC article.
-
Performance and use of HSV type-specific serology test kits.Herpes. 2002 Jul;9(2):38-45. Herpes. 2002. PMID: 12106510 Review.
-
Herpes serology for dermatologists.Arch Dermatol. 2000 Sep;136(9):1158-61. doi: 10.1001/archderm.136.9.1158. Arch Dermatol. 2000. PMID: 10987876 Review.
Cited by
-
Evaluating HIV Prevention Programs: Herpes Simplex Virus Type 2 Antibodies as Biomarker for Sexual Risk Behavior in Young Adults in Resource-Poor Countries.PLoS One. 2015 May 26;10(5):e0128370. doi: 10.1371/journal.pone.0128370. eCollection 2015. PLoS One. 2015. PMID: 26010772 Free PMC article.
-
Evaluation of Herpes Simplex Virus Type 2 Serological Tests for Use With Dried Blood Spots in Kenya.Sex Transm Dis. 2017 Feb;44(2):101-103. doi: 10.1097/OLQ.0000000000000557. Sex Transm Dis. 2017. PMID: 28081046 Free PMC article.
-
Herpes diagnostic tests and their use.Curr Infect Dis Rep. 2012 Apr;14(2):175-84. doi: 10.1007/s11908-012-0241-0. Curr Infect Dis Rep. 2012. PMID: 22311664
-
Detection, quantification and genotyping of Herpes Simplex Virus in cervicovaginal secretions by real-time PCR: a cross sectional survey.Virol J. 2005 Aug 11;2:61. doi: 10.1186/1743-422X-2-61. Virol J. 2005. PMID: 16095535 Free PMC article.
-
Comparison of focus HerpesSelect and Kalon HSV-2 gG2 ELISA serological assays to detect herpes simplex virus type 2 antibodies in a South African population.Sex Transm Infect. 2010 Feb;86(1):46-50. doi: 10.1136/sti.2009.036541. Epub 2009 Oct 16. Sex Transm Infect. 2010. PMID: 19837726 Free PMC article.
References
-
- Ashley, R. L. 2002. Performance and use of HSV type specific serology test kits. Herpes 9:38-45. - PubMed
-
- Ashley, R. L., E. Krantz, and A. Wald. 2003. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex. Transm. Dis. 30:310-314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

