J protein cochaperone of the mitochondrial inner membrane required for protein import into the mitochondrial matrix
- PMID: 14605210
- PMCID: PMC283508
- DOI: 10.1073/pnas.1936150100
J protein cochaperone of the mitochondrial inner membrane required for protein import into the mitochondrial matrix
Abstract
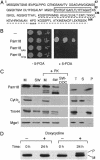
The major Hsp70 of the mitochondrial matrix (Ssc1 in yeast) is critically important for the translocation of proteins from the cytosol, across the mitochondrial inner membrane, and into the matrix. Tim44, a peripheral inner membrane protein with limited sequence similarity to the J domain of J-type cochaperones, tethers Ssc1 to the import channel. Here we report that, unlike a J protein, Tim44 does not stimulate the ATPase activity of Ssc1, nor does it affect the stimulation by either a known mitochondrial J protein or a peptide substrate. Thus, we conclude that Tim44 does not function as a J protein cochaperone of Ssc1; rather, it tethers Ssc1 to the import channel through interactions independent of those critical for J protein function. However, a previously unstudied essential gene, PAM18, encodes an 18-kDa protein that contains a J domain and is localized to the mitochondrial inner membrane. Pam18 stimulates the ATPase activity of Ssc1; depletion of Pam18 in vivo disrupts import of proteins into the mitochondrial matrix. We propose that Pam18 is the J protein partner for Ssc1 at the import channel and is critical for Ssc1's function in protein import.
Figures

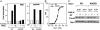
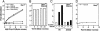
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

