Possible involvement of leaf gibberellins in the clock-controlled expression of XSP30, a gene encoding a xylem sap lectin, in cucumber roots
- PMID: 14605217
- PMCID: PMC300732
- DOI: 10.1104/pp.103.030742
Possible involvement of leaf gibberellins in the clock-controlled expression of XSP30, a gene encoding a xylem sap lectin, in cucumber roots
Abstract
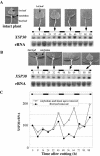
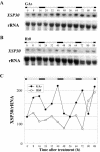
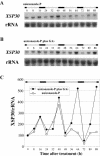
Root-produced organic compounds in xylem sap, such as hormones and amino acids, are known to be important in plant development. Recently, biochemical approaches have revealed the identities of several xylem sap proteins, but the biological functions and the regulation of the production of these proteins are not fully understood. XYLEM SAP PROTEIN 30 kD (XSP30), which is specifically expressed in the roots of cucumber (Cucumis sativus), encodes a lectin and is hypothesized as affecting the development of above-ground organs. In this report, we demonstrate that XSP30 gene expression and the level of XSP30 protein fluctuate in a diurnal rhythm in cucumber roots. The rhythmic gene expression continues for at least two or three cycles, even under continuous light or dark conditions, demonstrating that the expression of this gene is controlled by a circadian clock. Removal of mature leaves or treatment of shoots with uniconazole-P, an inhibitor of gibberellic acid (GA) biosynthesis, dampens the amplitude of the rhythmic expression; the application of GA negates these effects. These results suggest that light signals perceived by above-ground organs, as well as GA that is produced, possibly, in mature leaves, are important for the rhythmic expression of XSP30 in roots. This is the first demonstration of the regulation of the expression of a clock-controlled gene by GA.
Figures




Similar articles
-
Organic substances in xylem sap delivered to above-ground organs by the roots.J Plant Res. 2006 May;119(3):179-87. doi: 10.1007/s10265-005-0257-8. Epub 2006 Jan 28. J Plant Res. 2006. PMID: 16733632 Review.
-
cDNA cloning of a novel lectin-like xylem sap protein and its root-specific expression in cucumber.Plant Cell Physiol. 1999 Nov;40(11):1177-81. doi: 10.1093/oxfordjournals.pcp.a029504. Plant Cell Physiol. 1999. PMID: 10635119
-
Vascular tissue-specific gene expression of xylem sap glycine-rich proteins in root and their localization in the walls of metaxylem vessels in cucumber.Plant Cell Physiol. 2000 May;41(5):627-38. doi: 10.1093/pcp/41.5.627. Plant Cell Physiol. 2000. PMID: 10929946
-
Gibberellin Is Involved in Inhibition of Cucumber Growth and Nitrogen Uptake at Suboptimal Root-Zone Temperatures.PLoS One. 2016 May 23;11(5):e0156188. doi: 10.1371/journal.pone.0156188. eCollection 2016. PLoS One. 2016. PMID: 27213554 Free PMC article.
-
Differential responses of plasma membrane aquaporins in mediating water transport of cucumber seedlings under osmotic and salt stresses.Plant Cell Environ. 2015 Mar;38(3):461-73. doi: 10.1111/pce.12319. Epub 2014 Apr 14. Plant Cell Environ. 2015. PMID: 24601940
Cited by
-
High temperatures promote the uptake of hydrophobic pollutants by Cucurbita pepo via altered gene expression levels of major latex-like proteins.J Pestic Sci. 2020 May 20;45(2):75-80. doi: 10.1584/jpestics.D19-065. J Pestic Sci. 2020. PMID: 32508513 Free PMC article.
-
Xylem sap in cotton contains proteins that contribute to environmental stress response and cell wall development.Funct Integr Genomics. 2015 Jan;15(1):17-26. doi: 10.1007/s10142-014-0395-y. Epub 2014 Aug 28. Funct Integr Genomics. 2015. PMID: 25163431
-
A major latex-like protein is a key factor in crop contamination by persistent organic pollutants.Plant Physiol. 2013 Apr;161(4):2128-35. doi: 10.1104/pp.112.213645. Epub 2013 Feb 12. Plant Physiol. 2013. PMID: 23404917 Free PMC article.
-
Presence of a basic secretory protein in xylem sap and shoots of poplar in winter and its physicochemical activities against winter environmental conditions.J Plant Res. 2019 Sep;132(5):655-665. doi: 10.1007/s10265-019-01123-9. Epub 2019 Jul 9. J Plant Res. 2019. PMID: 31289959
-
Organic substances in xylem sap delivered to above-ground organs by the roots.J Plant Res. 2006 May;119(3):179-87. doi: 10.1007/s10265-005-0257-8. Epub 2006 Jan 28. J Plant Res. 2006. PMID: 16733632 Review.
References
-
- Abd-el Baki GK, Siefritz F, Man HM, Weiner H, Kaldenhoff R, Kaiser WM (2000) Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ 23: 515-521
-
- Bangerth F (1994) Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta 194: 439-442
-
- Bernier G (1988) The control of floral evocation and morphogenesis. Annu Rev Plant Physiol Plant Mol Biol 39: 175-219
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

