Impaired induction of the jasmonate pathway in the rice mutant hebiba
- PMID: 14605232
- PMCID: PMC300735
- DOI: 10.1104/pp.103.027490
Impaired induction of the jasmonate pathway in the rice mutant hebiba
Abstract
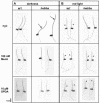
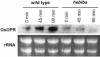
The elongation of rice (Oryza sativa) coleoptiles is inhibited by light, and this photoinhibition was used to screen for mutants with impaired light response. In one of the isolated mutants, hebiba, coleoptile elongation was stimulated in the presence of red light, but inhibited in the dark. Light responses of endogenous indolyl-3-acetic acid and abscisic acid were identical between the wild type and the mutant. In contrast, the wild type showed a dramatic increase of jasmonate heralded by corresponding increases in the content of its precursor o-phytodienoic acid, whereas both compounds were not detectable in the mutant. The jasmonate response to wounding was also blocked in the mutant. The mutant phenotype was rescued by addition of exogenous methyl jasmonate and o-phytodienoic acid. Moreover, the expression of O. sativa 12-oxophytodienoic acid reductase, an early gene of jasmonic acid-synthesis, is induced by red light in the wild type, but not in the mutant. This evidence suggests a novel role for jasmonates in the light response of growth, and we discuss a cross-talk between jasmonate and auxin signaling. In addition, hebiba represents the first rice mutant in which the induction of the jasmonate pathway is impaired providing a valuable tool to study the role of jasmonates in Graminean development.
Figures





References
-
- Agrawal GK, Rakwal R, Jwa N-S, Han K-S, Agrawal VP (2002) Molecular cloning and mRNA expression analysis of the first rice jasmonate biosynthetic pathway gene allene oxide synthase. Plant Physiol Biochem 40: 771-782
-
- Biswas KK, Neumann R, Haga K, Yatoh O, Iino M (2003) Photomorphogenesis of rice seedlings: a mutant impaired in phytochrome-mediated inhibition of coleoptile growth. Plant Cell Physiol 44: 242-254 - PubMed
-
- Brummer B, Bertl A, Potrykus I, Felle H, Parish RW (1985) Evidence that fusicoccon and indole-3-acetic acid induce cytosolic acidification of Zea mays cells. FEBS Lett 189: 109-114
-
- Clouse SD (2001) Integration of light and brassinosteroid signals in etiolated seedling growth. Trends Plant Sci 6: 443-445 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

