Inhibition of fibrocyte differentiation by serum amyloid P
- PMID: 14607961
- PMCID: PMC4482350
- DOI: 10.4049/jimmunol.171.10.5537
Inhibition of fibrocyte differentiation by serum amyloid P
Abstract
Wound healing and the dysregulated events leading to fibrosis both involve the proliferation and differentiation of fibroblasts and the deposition of extracellular matrix. Whether these fibroblasts are locally derived or from a circulating precursor population is unclear. Fibrocytes are a distinct population of fibroblast-like cells derived from peripheral blood monocytes that enter sites of tissue injury to promote angiogenesis and wound healing. We have found that CD14(+) peripheral blood monocytes cultured in the absence of serum or plasma differentiate into fibrocytes within 72 h. We purified the factor in serum and plasma that prevents the rapid appearance of fibrocytes, and identified it as serum amyloid P (SAP). Purified SAP inhibits fibrocyte differentiation at levels similar to those found in plasma, while depleting SAP reduces the ability of plasma to inhibit fibrocyte differentiation. Compared with sera from healthy individuals and patients with rheumatoid arthritis, sera from patients with scleroderma and mixed connective tissue disease, two systemic fibrotic diseases, were less able to inhibit fibrocyte differentiation in vitro and had correspondingly lower serum levels of SAP. These results suggest that low levels of SAP may thus augment pathological processes leading to fibrosis. These data also suggest mechanisms to inhibit fibrosis in chronic inflammatory conditions, or conversely to promote wound healing.
Figures
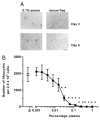

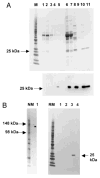
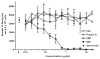
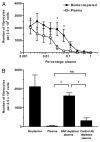

References
-
- Clark RA. Fibrin and wound healing. Ann NY Acad Sci. 2001;936:355. - PubMed
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60. - PubMed
-
- Akbar AN, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T cell death. Immunol Today. 1997;18:72. - PubMed
-
- Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199. - PubMed
-
- Akbar AN, Salmon M. Chronic inflammatory states maintained by abnormal stromal microenvironments. Immunology. 1996;89:51.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

