Mexiletine block of wild-type and inactivation-deficient human skeletal muscle hNav1.4 Na+ channels
- PMID: 14608007
- PMCID: PMC1664796
- DOI: 10.1113/jphysiol.2003.054973
Mexiletine block of wild-type and inactivation-deficient human skeletal muscle hNav1.4 Na+ channels
Abstract
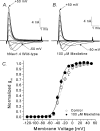
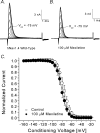
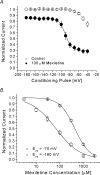
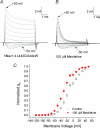
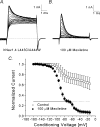
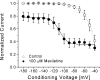
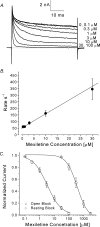
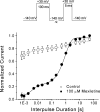
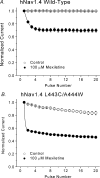
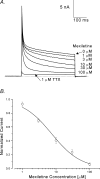
Mexiletine is a class 1b antiarrhythmic drug used for ventricular arrhythmias but is also found to be effective for paramyotonia congenita, potassium-aggravated myotonia, long QT-3 syndrome, and neuropathic pain. This drug elicits tonic block of Na(+) channels when cells are stimulated infrequently and produces additional use-dependent block during repetitive pulses. We examined the state-dependent block by mexiletine in human skeletal muscle hNav1.4 wild-type and inactivation-deficient mutant Na(+) channels (hNav1.4-L443C/A444W) expressed in HEK293t cells with a beta1 subunit. The 50% inhibitory concentrations (IC(50)) for the inactivated-state block and the resting-state block of wild-type Na(+) channels by mexiletine were measured as 67.8 +/- 7.0 microm and 431.2 +/- 9.4 microm, respectively (n= 5). In contrast, the IC(50) for the block of open inactivation-deficient mutant channels at +30 mV by mexiletine was 3.3 +/- 0.1 microm (n= 5), which was within the therapeutic plasma concentration range (2.8-11 microm). Estimated on- and off-rates for the open-state block by mexiletine at +30 mV were 10.4 microm(-1) s(-1) and 54.4 s(-1), respectively. Use-dependent block by mexiletine was greater in inactivation-deficient mutant channels than in wild-type channels during repetitive pulses. Furthermore, the IC(50) values for the block of persistent late hNav1.4 currents in chloramine-T-pretreated cells by mexiletine was 7.5 +/- 0.8 microm (n= 5) at +30 mV. Our results together support the hypothesis that the in vivo efficacy of mexiletine is primarily due to the open-channel block of persistent late Na(+) currents, which may arise during various pathological conditions.
Figures
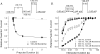

References
-
- Cannon SC. An expanding view of the molecular basis of familial periodic paralysis. Neuromuscular Disorders. 2002;12:533–543. - PubMed
-
- Cannon SC, Strittmatter SM. Functional expression of sodium channel mutations identified in families with periodic paralysis. Neuron. 1993;10:317–326. - PubMed
-
- Catterall WA, Mackie K. Local anesthetics. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. New York: Macmillan Publishing Co.; 2001. pp. 367–384.
-
- Chabal C, Jacobson L, Mariano A, Chaney E, Britell CW. The use of oral mexiletine for the treatment of pain after peripheral nerve injury. Anesthesiology. 1992;76:513–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

