Different flecainide sensitivity of hNav1.4 channels and myotonic mutants explained by state-dependent block
- PMID: 14608015
- PMCID: PMC1664778
- DOI: 10.1113/jphysiol.2003.046995
Different flecainide sensitivity of hNav1.4 channels and myotonic mutants explained by state-dependent block
Erratum in
- J Physiol. 2004 Mar 16;555(Pt 3):851. Luca, Annamaria D E [corrected to De Luca, Annamaria]
Abstract
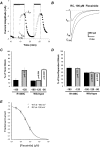
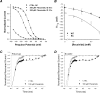
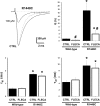
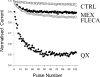
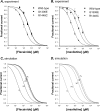
Flecainide, a class IC antiarrhythmic, was shown to improve myotonia caused by sodium channel mutations in situations where the class IB antiarrhythmic drug mexiletine was less efficient. Yet little is known about molecular interactions between flecainide and human skeletal muscle sodium (hNa(v)1.4) channels. Whole-cell sodium currents (I(Na)) were recorded in tsA201 cells expressing wild-type (WT) and mutant hNa(v)1.4 channels (R1448C, paramyotonia congenita; G1306E, potassium-aggravated myotonia). At a holding potential (HP) of -120 mV, flecainide use-dependently blocked WT and G1306E I(Na) equally but was more potent on R1448C channels. For WT, the extent of block depended on a holding voltage more negative than the activation threshold, being greater at -90 mV as compared to -120 and -180 mV. This behaviour was exacerbated by the R1448C mutation since block at -120 mV was greater than that at -180 mV. Thus flecainide can bind to inactivated sodium channels in the absence of channel opening. Nevertheless, all the channels showed the same closed-state affinity constant (K(R) approximately 480 microM) and the same inactivated-state affinity constant (K(I) approximately 18 microM). Simulations according to the modulated receptor hypothesis mimic the voltage-dependent block of WT and mutant channels by flecainide and mexiletine. All the results suggest similar blocking mechanisms for the two drugs. Yet, since flecainide exerts use-dependent block at lower frequency than mexiletine, it may exhibit greater benefit in all myotonic syndromes. Moreover, flecainide blocks hNa(v)1.4 channel mutants with a rightward shift of availability voltage dependence more specifically than mexiletine, owing to a lower K(R)/K(I) ratio. This study offers a pharmacogenetic strategy to better address treatment in individual myotonic patients.
Figures

References
-
- Abriel H, Wehrens XHT, Benhorin J, Kerem B, Kass RS. Molecular pharmacology of the sodium channel mutation D1790G linked to the long-QT syndrome. Circulation. 2000;102:921–925. - PubMed
-
- American Heart Association. International guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 6: Advanced Cardiovascular Life Support: Section 5: Pharmacology I: Agents for Arrhythmias. Circulation. 2000;102:I112–I128. - PubMed
-
- Anno T, Hondeghem LM. Interactions of flecainide with guinea pig cardiac sodium channels. Importance of activation unblocking to the voltage-dependence of recovery. Circ Res. 1990;66:789–803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

