Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes
- PMID: 14610020
- PMCID: PMC2229598
- DOI: 10.1085/jgp.200308899
Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes
Abstract
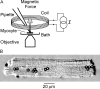
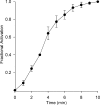
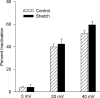
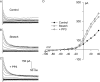
Osmotic swelling of cardiac myocytes and other types of cells activates an outwardly rectifying, tamoxifen-sensitive Cl- current, ICl,swell, but it is unclear whether Cl- currents also are activated by direct mechanical stretch. We tested whether specific stretch of beta1-integrin activates a Cl- current in rabbit left ventricular myocytes. Paramagnetic beads (4.5-microm diameter) coated with mAb to beta1-integrin were applied to the surface of myocytes and pulled upward with an electromagnet while recording whole-cell current. In solutions designed to isolate anion currents, beta1-integrin stretch elicited an outwardly rectifying Cl- current with biophysical and pharmacological properties similar to those of ICl,swell. Stretch-activated Cl- current activated slowly (t1/2 = 3.5 +/- 0.1 min), partially inactivated at positive voltages, reversed near ECl, and was blocked by 10 microM tamoxifen. When stretch was terminated, 64 +/- 8% of the stretch-induced current reversed within 10 min. Mechanotransduction involved protein tyrosine kinase. Genistein (100 microM), a protein tyrosine kinase inhibitor previously shown to suppress ICl,swell in myocytes, inhibited stretch-activated Cl- current by 62 +/- 6% during continued stretch. Because focal adhesion kinase and Src are known to be activated by cell swelling, mechanical stretch, and clustering of integrins, we tested whether these tyrosine kinases mediated the response to beta1-integrin stretch. PP2 (10 microM), a selective blocker of focal adhesion kinase and Src, fully inhibited the stretch-activated Cl- current as well as part of the background Cl- current, whereas its inactive analogue PP3 (10 microM) had no significant effect. In addition to activating Cl- current, stretch of beta1-integrin also appeared to activate a nonselective cation current and to suppress IK1. Integrins are the primary mechanical link between the extracellular matrix and cytoskeleton. The present results suggest that integrin stretch may contribute to mechano-electric feedback in heart, modulate electrical activity, and influence the propensity for arrhythmogenesis.
Figures






References
-
- Babbitt, C.J., S.Y. Shai, A.E. Harpf, C.G. Pham, and R.S. Ross. 2002. Modulation of integrins and integrin signaling molecules in the pressure-loaded murine ventricle. Histochem. Cell Biol. 118:431–439. - PubMed
-
- Baumgarten, C.M., and H.F. Clemo. 2003. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog. Biophys. Mol. Biol. 82:25–42. - PubMed
-
- Bendall, J.K., C. Heymes, P. Ratajczak, and J.L. Samuel. 2002. Extracellular matrix and cardiac remodelling. Arch. Mal. Coeur Vaiss. 95:1226–1229. - PubMed
-
- Bénitah, J.P., A.M. Gomez, C. Delgado, P. Lorente, and W.J. Lederer. 1997. A chloride current component induced by hypertrophy in rat ventricular myocytes. Am. J. Physiol. 272:H2500–H2506. - PubMed
-
- Borg, T.K., E.C. Goldsmith, R. Price, W. Carver, L. Terracio, and A.M. Samarel. 2000. Specialization at the Z line of cardiac myocytes. Cardiovasc. Res. 46:277–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

