A core function for p120-catenin in cadherin turnover
- PMID: 14610055
- PMCID: PMC2173649
- DOI: 10.1083/jcb.200307111
A core function for p120-catenin in cadherin turnover
Abstract
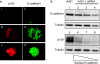
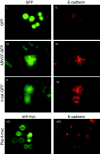
p120-catenin stabilizes epithelial cadherin (E-cadherin) in SW48 cells, but the mechanism has not been established. Here, we show that p120 acts at the cell surface to control cadherin turnover, thereby regulating cadherin levels. p120 knockdown by siRNA expression resulted in dose-dependent elimination of epithelial, placental, neuronal, and vascular endothelial cadherins, and complete loss of cell-cell adhesion. ARVCF and delta-catenin were functionally redundant, suggesting that proper cadherin-dependent adhesion requires the presence of at least one p120 family member. The data reveal a core function of p120 in cadherin complexes, and strongly predict a dose-dependent loss of E-cadherin in tumors that partially or completely down-regulate p120.
Figures





Comment in
-
Traffic control: p120-catenin acts as a gatekeeper to control the fate of classical cadherins in mammalian cells.J Cell Biol. 2003 Nov 10;163(3):437-40. doi: 10.1083/jcb.200310090. J Cell Biol. 2003. PMID: 14610049 Free PMC article. Review.
References
-
- Anastasiadis, P.Z., and A.B. Reynolds. 2000. The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113:1319–1334. - PubMed
-
- Anastasiadis, P.Z., and A.B. Reynolds. 2001. Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13:604–610. - PubMed
-
- Anastasiadis, P.Z., S.Y. Moon, M.A. Thoreson, D.J. Mariner, H.C. Crawford, Y. Zheng, and A.B. Reynolds. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2:637–644. - PubMed
-
- Baki, L., P. Marambaud, S. Efthimiopoulos, A. Georgakopoulos, P. Wen, W. Cui, J. Shioi, E. Koo, M. Ozawa, V.L. Friedrich, Jr., and N.K. Robakis. 2001. Presenilin-1 binds cytoplasmic epithelial cadherin, inhibits cadherin/p120 association, and regulates stability and function of the cadherin/catenin adhesion complex. Proc. Natl. Acad. Sci. USA. 98:2381–2386. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

