Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites
- PMID: 14610058
- PMCID: PMC2173641
- DOI: 10.1083/jcb.200302157
Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites
Abstract
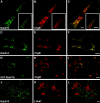
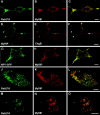
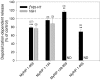
The GTPase Rab27A interacts with myosin-VIIa and myosin-Va via MyRIP or melanophilin and mediates melanosome binding to actin. Here we show that Rab27A and MyRIP are associated with secretory granules (SGs) in adrenal chromaffin cells and PC12 cells. Overexpression of Rab27A, GTPase-deficient Rab27A-Q78L, or MyRIP reduced secretory responses of PC12 cells. Amperometric recordings of single adrenal chromaffin cells revealed that Rab27A-Q78L and MyRIP reduced the sustained component of release. Moreover, these effects on secretion were partly suppressed by the actin-depolymerizing drug latrunculin but strengthened by jasplakinolide, which stabilizes the actin cortex. Finally, MyRIP and Rab27A-Q78L restricted the motion of SGs in the subplasmalemmal region of PC12 cells, as measured by evanescent-wave fluorescence microscopy. In contrast, the Rab27A-binding domain of MyRIP and a MyRIP construct that interacts with myosin-Va but not with actin increased the mobility of SGs. We propose that Rab27A and MyRIP link SGs to F-actin and control their motion toward release sites through the actin cortex.
Figures










References
-
- Bhakdi, S., U. Weller, I. Walev, E. Martin, D. Jonas, and M. Palmer. 1993. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med. Microbiol. Immunol. (Berl.). 182:167–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

