Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA
- PMID: 14610062
- PMCID: PMC2173651
- DOI: 10.1083/jcb.200309042
Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA
Abstract
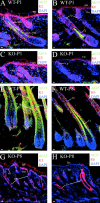
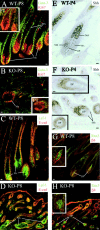
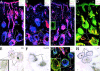
Using conditional gene targeting in mice, we show that BMP receptor IA is essential for the differentiation of progenitor cells of the inner root sheath and hair shaft. Without BMPRIA activation, GATA-3 is down-regulated and its regulated control of IRS differentiation is compromised. In contrast, Lef1 is up-regulated, but its regulated control of hair differentiation is still blocked, and BMPRIA-null follicles fail to activate Lef1/beta-catenin-regulated genes, including keratin genes. Wnt-mediated transcriptional activation can be restored by transfecting BMPRIA-null keratinocytes with a constitutively activated beta-catenin. This places the block downstream from Lef1 expression but upstream from beta-catenin stabilization. Because mice lacking the BMP inhibitor Noggin fail to express Lef1, our findings support a model, whereby a sequential inhibition and then activation of BMPRIA is necessary to define a band of hair progenitor cells, which possess enough Lef1 and stabilized beta-catenin to activate the hair specific keratin genes and generate the hair shaft.
Figures





Similar articles
-
Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation.Development. 1999 Oct;126(20):4557-68. doi: 10.1242/dev.126.20.4557. Development. 1999. PMID: 10498690
-
BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice.Development. 2004 Apr;131(8):1825-33. doi: 10.1242/dev.01079. Epub 2004 Mar 17. Development. 2004. PMID: 15084466
-
Links between signal transduction, transcription and adhesion in epithelial bud development.Nature. 2003 Mar 20;422(6929):317-22. doi: 10.1038/nature01458. Nature. 2003. PMID: 12646922 Free PMC article.
-
Bone morphogenetic proteins and their antagonists in skin and hair follicle biology.J Invest Dermatol. 2003 Jan;120(1):36-47. doi: 10.1046/j.1523-1747.2003.12002.x. J Invest Dermatol. 2003. PMID: 12535196 Review.
-
Monstrous attempts at adnexogenesis: regulating hair follicle progenitors through Sonic hedgehog signaling.Curr Opin Genet Dev. 2001 Oct;11(5):541-6. doi: 10.1016/s0959-437x(00)00230-6. Curr Opin Genet Dev. 2001. PMID: 11532396 Review.
Cited by
-
The bone morphogenetic protein receptor-1A pathway is required for lactogenic differentiation of mammary epithelial cells in vitro.In Vitro Cell Dev Biol Anim. 2012 Jun;48(6):377-84. doi: 10.1007/s11626-012-9522-z. Epub 2012 Jun 23. In Vitro Cell Dev Biol Anim. 2012. PMID: 22729646 Free PMC article.
-
Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors.Dev Cell. 2012 Nov 13;23(5):981-94. doi: 10.1016/j.devcel.2012.10.013. Dev Cell. 2012. PMID: 23153495 Free PMC article.
-
Its written all over your face: The molecular and physiological consequences of aging skin.Mech Ageing Dev. 2020 Sep;190:111315. doi: 10.1016/j.mad.2020.111315. Epub 2020 Jul 15. Mech Ageing Dev. 2020. PMID: 32681843 Free PMC article. Review.
-
The mammary bud as a skin appendage: unique and shared aspects of development.J Mammary Gland Biol Neoplasia. 2006 Oct;11(3-4):187-203. doi: 10.1007/s10911-006-9029-x. J Mammary Gland Biol Neoplasia. 2006. PMID: 17111222 Review.
-
Tooth, hair and claw: comparing epithelial stem cell niches of ectodermal appendages.Exp Cell Res. 2014 Jul 15;325(2):96-103. doi: 10.1016/j.yexcr.2014.02.003. Epub 2014 Feb 14. Exp Cell Res. 2014. PMID: 24530577 Free PMC article. Review.
References
-
- Ahn, K., Y. Mishina, M.C. Hanks, R.R. Behringer, and E.B. Crenshaw, III. 2001. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 128:4449–4461. - PubMed
-
- Alonso, L., and E. Fuchs. 2003. Stem cells in the skin: waste not, Wnt not. Genes Dev. 17:1189–1200. - PubMed
-
- Andl, T., S.T. Reddy, T. Gaddapara, and S.E. Millar. 2002. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2:643–653. - PubMed
-
- Balciunaite, G., M.P. Keller, E. Balciunaite, L. Piali, S. Zuklys, Y.D. Mathieu, J. Gill, R. Boyd, D.J. Sussman, and G.A. Hollander. 2002. Wnt glycoproteins regulate the expression of Foxn1, the gene defective in nude mice. Nat. Immunol. 3:1102–1108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

