Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements
- PMID: 14610063
- PMCID: PMC2173654
- DOI: 10.1083/jcb.200302152
Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements
Abstract
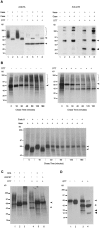
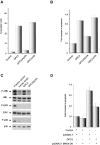
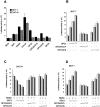
Glypican (GPC)-3 inhibits cell proliferation and regulates cell survival during development. This action is demonstrated by GPC3 loss-of-function mutations in humans and mice. Here, we show that the GPC3 core protein is processed by a furinlike convertase. This processing is essential for GPC3 modulating Wnt signaling and cell survival in vitro and for supporting embryonic cell movements in zebrafish. The processed GPC3 core protein is necessary and sufficient for the cell-specific induction of apoptosis, but in vitro effects on canonical and noncanonical Wnt signaling additionally require substitution of the core protein with heparan sulfate. Wnt 5A physically associates only with processed GPC3, and only a form of GPC3 that can be processed by a convertase is able to rescue epiboly and convergence/extension movements in GPC3 morphant embryos. Our data imply that the Simpson-Golabi-Behmel syndrome may in part result from a loss of GPC3 controls on Wnt signaling, and suggest that this function requires the cooperation of both the protein and the heparan sulfate moieties of the proteoglycan.
Figures






Similar articles
-
Processing by convertases is not required for glypican-3-induced stimulation of hepatocellular carcinoma growth.J Biol Chem. 2005 Dec 16;280(50):41201-6. doi: 10.1074/jbc.M507004200. Epub 2005 Oct 14. J Biol Chem. 2005. PMID: 16227623
-
Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis.Development. 2003 May;130(10):2129-38. doi: 10.1242/dev.00435. Development. 2003. PMID: 12668627
-
The loss of glypican-3 induces alterations in Wnt signaling.J Biol Chem. 2005 Jan 21;280(3):2116-25. doi: 10.1074/jbc.M410090200. Epub 2004 Nov 10. J Biol Chem. 2005. PMID: 15537637
-
Glypicans and the biology of renal malformations.Pediatr Nephrol. 2001 Mar;16(3):302-6. doi: 10.1007/s004670000530. Pediatr Nephrol. 2001. PMID: 11322381 Review.
-
Simpson Golabi Behmel syndrome: progress toward understanding the molecular basis for overgrowth, malformation, and cancer predisposition.Mol Genet Metab. 2001 Apr;72(4):279-86. doi: 10.1006/mgme.2001.3150. Mol Genet Metab. 2001. PMID: 11286501 Review.
Cited by
-
An Unbiased Mass Spectrometry Approach Identifies Glypican-3 as an Interactor of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) and Low Density Lipoprotein Receptor (LDLR) in Hepatocellular Carcinoma Cells.J Biol Chem. 2016 Nov 18;291(47):24676-24687. doi: 10.1074/jbc.M116.746883. Epub 2016 Oct 7. J Biol Chem. 2016. PMID: 27758865 Free PMC article.
-
Glypican-3-mediated oncogenesis involves the Insulin-like growth factor-signaling pathway.Carcinogenesis. 2008 Jul;29(7):1319-26. doi: 10.1093/carcin/bgn091. Epub 2008 Apr 15. Carcinogenesis. 2008. PMID: 18413366 Free PMC article.
-
Hedgehog on the Move: Glypican-Regulated Transport and Gradient Formation in Drosophila.Cells. 2024 Feb 27;13(5):418. doi: 10.3390/cells13050418. Cells. 2024. PMID: 38474382 Free PMC article. Review.
-
Therapeutic potential of targeting glypican-3 in hepatocellular carcinoma.Anticancer Agents Med Chem. 2011 Jul;11(6):543-8. doi: 10.2174/187152011796011109. Anticancer Agents Med Chem. 2011. PMID: 21554204 Free PMC article. Review.
-
Glypican-3 deficiency in liver cancer upregulates MAPK/ERK pathway but decreases cell proliferation.Am J Cancer Res. 2024 Jul 15;14(7):3348-3371. doi: 10.62347/TTNY4279. eCollection 2024. Am J Cancer Res. 2024. PMID: 39113871 Free PMC article.
References
-
- Baeg, G.H., X. Lin, N. Khare, S. Baumgartner, and N. Perrimon. 2001. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 128:87–94. - PubMed
-
- Cano-Gauci, D.F., H.H. Song, H. Yang, C. McKerlie, B. Choo, W. Shi, R. Pullano, T.D. Piscione, S. Grisaru, S. Soon, et al. 1999. Glypican-3–deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson–Golabi–Behmel syndrome. J. Cell Biol. 146:255–264. - PMC - PubMed
-
- Chen, R.L., and A.D. Lander. 2001. Mechanisms underlying preferential assembly of heparan sulfate on glypican-1. J. Biol. Chem. 276:7507–7517. - PubMed
-
- Chiao, E., P. Fisher, L. Crisponi, M. Deiana, I. Dragatsis, D. Schlessinger, G. Pilia, and A. Efstratiadis. 2002. Overgrowth of a mouse model of the Simpson–Golabi–Behmel syndrome is independent of IGF signaling. Dev. Biol. 243:185–206. - PubMed
-
- Creemers, J.W., D. Ines Dominguez, E. Plets, L. Serneels, N.A. Taylor, G. Multhaup, K. Craessaerts, W. Annaert, and B. De Strooper. 2001. Processing of beta-secretase by furin and other members of the proprotein convertase family. J. Biol. Chem. 276:4211–4217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

