Modulation of minute virus of mice cytotoxic activities through site-directed mutagenesis within the NS coding region
- PMID: 14610171
- PMCID: PMC262581
- DOI: 10.1128/jvi.77.23.12466-12478.2003
Modulation of minute virus of mice cytotoxic activities through site-directed mutagenesis within the NS coding region
Abstract
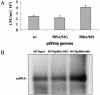
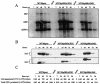
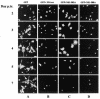
Late in infection, parvovirus minute virus of mice (MVMp) induces the lysis of mouse A9 fibroblasts. This effect depends on the large nonstructural phosphoprotein NS1, which plays in addition a major role in viral DNA replication and progeny particle production. Since the NS1 C-terminal region is subjected to late phosphorylation events and protein kinase C (PKC) family members regulate NS1 replicative activities, the present study was conducted to determine the impact of PKCs on NS1 cytotoxic functions. To this end, we performed site-directed mutagenesis, substituting alanine residues for two consensus PKC-phosphorylation sites located within the NS1 C-terminal region, T585 and S588. Although these substitutions had no detectable effect on virus multiplication in a single-round infection, the NS1-585A mutant virus was significantly less toxic to A9 cells than wild-type MVMp, whereas the NS1-588A mutant virus was endowed with a higher killing potential. These alterations correlated with specific changes in the late phosphorylation pattern of the mutant NS1 proteins compared to the wild-type polypeptide. Since the mutations introduced in this region of the viral genome also made changes in the minor nonstructural protein NS2, a contribution of this polypeptide to the above-mentioned phenotypes of mutant viruses cannot be excluded at present. However, the involvement of NS1 in these phenotypes was directly supported by the respective reduced and enhanced capacity of NS1-585A and NS1-588A recombinant proteins for inducing morphological alterations and cell detachment in transfected A9 cultures. Altogether, these data suggest that late-occurring phosphorylation of NS1 specifically regulates the cytotoxic functions of the viral product and that residues T585 and S588 contribute to this control in an antagonistic way.
Figures





References
-
- Brandenburger, A., D. Legendre, B. Avalosse, and J. Rommelaere. 1990. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology 174:576-584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

