Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response
- PMID: 14610206
- PMCID: PMC262578
- DOI: 10.1128/jvi.77.23.12852-12864.2003
Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response
Abstract
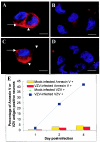
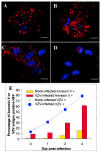
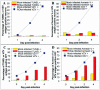
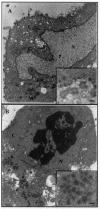
The induction of apoptosis or programmed cell death in virus-infected cells is an important antiviral defense mechanism of the host, and some herpesviruses have evolved strategies to modulate apoptosis in order to enhance their survival and spread. In this study, we examined the ability of varicella-zoster virus (VZV) to induce apoptosis in primary human dorsal root ganglion neurons and primary human foreskin fibroblasts (HFFs). Three independent methods (annexin V, TUNEL [terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling] staining, and electron microscopy) were used to assess apoptosis in these cells on days 1, 2, and 4 postinoculation. By all three methods, apoptosis was readily detected in VZV-infected HFFs. In stark contrast, apoptosis was not detected during productive VZV infection of neurons. The low-passage clinical isolate Schenke and the tissue culture-adapted ROka strain both induced apoptosis in HFFs but not in neurons, suggesting that this cell-type-specific apoptotic phenotype was not VZV strain specific. These data show that the regulation of apoptosis differs markedly between HFFs and neurons during productive VZV infection. Inhibition of apoptosis during infection of neurons may play a significant role in the establishment, maintenance, and reactivation of latent infection by promoting survival of these postmitotic cells.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

