Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor beta1
- PMID: 14612410
- PMCID: PMC262670
- DOI: 10.1128/MCB.23.23.8691-8703.2003
Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor beta1
Abstract
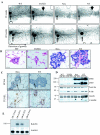
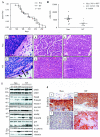
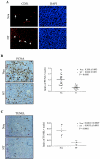
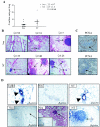
To determine if Neu is dominant over transforming growth factor beta (TGF-beta), we crossed mouse mammary tumor virus (MMTV)-Neu mice with MMTV-TGF-beta1(S223/225) mice expressing active TGF-beta1 in the mammary gland. Bigenic (NT) and Neu-induced mammary tumors developed with a similar latency. The bigenic tumors and their metastases were less proliferative than those occurring in MMTV-Neu mice. However, NT tumors exhibited less apoptosis and were more locally invasive and of higher histological grade. NT mice exhibited more circulating tumor cells and lung metastases than Neu mice, while NT tumors contained higher levels of phosphorylated (active) Smad2, Akt, mitogen-activated protein kinase (MAPK), and p38, as well as vimentin content and Rac1 activity in situ than tumors expressing Neu alone. Ex vivo, NT cells exhibited higher levels of P-Akt and P-MAPK than Neu cells. These were inhibited by the TGF-beta inhibitor-soluble TGF-beta type II receptor (TbetaRII:Fc), suggesting they were activated by autocrine TGF-beta. TGF-beta stimulated migration of Neu cells into surrounding matrix, while the soluble TGF-beta inhibitor abrogated motility and invasiveness of NT cells. These data suggest that (i) the antimitogenic and prometastatic effects of TGF-beta can exist simultaneously and (ii) Neu does not abrogate TGF-beta-mediated antiproliferative action but can synergize with TGF-beta in accelerating metastatic tumor progression.
Figures

References
-
- Adam, L., R. Vadlamudi, S. B. Kondapaka, J. Chernoff, J. Mendelsohn, and R. Kumar. 1998. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 273:28238-28246. - PubMed
-
- Atfi, A., S. Djelloul, E. Chastre, R. Davis, and C. Gespach. 1997. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J. Biol. Chem. 272:1429-1432. - PubMed
-
- Bakin, A. V., C. Rinehart, A. K. Tomlinson, and C. L. Arteaga. 2002. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 115:3193-3206. - PubMed
-
- Berking, C., R. Takemoto, H. Schaider, L. Showe, K. Satyamoorthy, P. Robbins, and M. Herlyn. 2001. Transforming growth factor-beta1 increases survival of human melanoma through stroma remodeling. Cancer Res. 61:8306-8316. - PubMed
-
- Bhowmick, N. A., R. Zent, M. Ghiassi, M. McDonnell, and H. L. Moses. 2001. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 276:46707-46713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
