The Cdk-activating kinase Cak1p promotes meiotic S phase through Ime2p
- PMID: 14612412
- PMCID: PMC262685
- DOI: 10.1128/MCB.23.23.8718-8728.2003
The Cdk-activating kinase Cak1p promotes meiotic S phase through Ime2p
Abstract
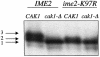
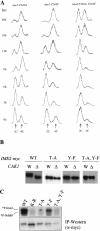
CAK1 encodes an essential protein kinase in Saccharomyces cerevisiae that is required for activation of the Cdc28p Cdk. CAK1 also has several CDC28-independent functions that are unique to meiosis. The earliest of these functions is to induce S phase, which is regulated differently in meiosis than in mitosis. In mitosis, Cdc28p controls its own S-phase-promoting activity by signaling the destruction of its inhibitor, Sic1p. In meiosis, Sic1p destruction is signaled by the meiosis-specific Ime2p protein kinase. Our data show that Cak1p is required to activate Ime2p through a mechanism that requires threonine 242 and tyrosine 244 in Ime2p's activation loop. This activation promotes autophosphorylation and accumulation of multiply phosphorylated forms of Ime2p during meiotic development. Consistent with Cak1p's role in activating Ime2p, cells lacking Cak1p are deficient in degrading Sic1p. Deletion of SIC1 or overexpression of IME2 can partially suppress the S-phase defect in cak1 mutant cells, suggesting that Ime2p is a key target of Cak1p regulation. These data show that Cak1p is required for the destruction of Sic1p in meiosis, as in mitosis, but in meiosis, it functions through a sporulation-specific kinase.
Figures
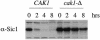

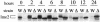



Similar articles
-
Phosphorylation of Ime2 regulates meiotic progression in Saccharomyces cerevisiae.J Biol Chem. 2006 Jul 7;281(27):18307-16. doi: 10.1074/jbc.M602349200. Epub 2006 May 9. J Biol Chem. 2006. PMID: 16684773
-
CAK1 promotes meiosis and spore formation in Saccharomyces cerevisiae in a CDC28-independent fashion.Mol Cell Biol. 2002 Jan;22(1):57-68. doi: 10.1128/MCB.22.1.57-68.2002. Mol Cell Biol. 2002. PMID: 11739722 Free PMC article.
-
Glucose inhibits meiotic DNA replication through SCFGrr1p-dependent destruction of Ime2p kinase.Mol Cell Biol. 2005 Jan;25(1):440-50. doi: 10.1128/MCB.25.1.440-450.2005. Mol Cell Biol. 2005. PMID: 15601864 Free PMC article.
-
Ime2p and Cdc28p: co-pilots driving meiotic development.J Cell Biochem. 2004 Aug 1;92(5):1025-33. doi: 10.1002/jcb.20131. J Cell Biochem. 2004. PMID: 15258924 Review.
-
The Ime2 protein kinase family in fungi: more duties than just meiosis.Mol Microbiol. 2011 Apr;80(1):1-13. doi: 10.1111/j.1365-2958.2011.07575.x. Epub 2011 Mar 1. Mol Microbiol. 2011. PMID: 21306447 Review.
Cited by
-
Protein Kinase Ime2 Is Required for Mycelial Growth, Conidiation, Osmoregulation, and Pathogenicity in Nematode-Trapping Fungus Arthrobotrys oligospora.Front Microbiol. 2020 Jan 14;10:3065. doi: 10.3389/fmicb.2019.03065. eCollection 2019. Front Microbiol. 2020. PMID: 31993040 Free PMC article.
-
CDK Regulation of Meiosis: Lessons from S. cerevisiae and S. pombe.Genes (Basel). 2020 Jun 29;11(7):723. doi: 10.3390/genes11070723. Genes (Basel). 2020. PMID: 32610611 Free PMC article. Review.
-
Ascospore formation in the yeast Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2005 Dec;69(4):565-84. doi: 10.1128/MMBR.69.4.565-584.2005. Microbiol Mol Biol Rev. 2005. PMID: 16339736 Free PMC article. Review.
-
Arg-Pro-X-Ser/Thr is a consensus phosphoacceptor sequence for the meiosis-specific Ime2 protein kinase in Saccharomyces cerevisiae.Biochemistry. 2007 Jan 9;46(1):271-8. doi: 10.1021/bi061858p. Biochemistry. 2007. PMID: 17198398 Free PMC article.
-
Ime1 and Ime2 are required for pseudohyphal growth of Saccharomyces cerevisiae on nonfermentable carbon sources.Mol Cell Biol. 2010 Dec;30(23):5514-30. doi: 10.1128/MCB.00390-10. Epub 2010 Sep 27. Mol Cell Biol. 2010. PMID: 20876298 Free PMC article.
References
-
- Chu, S., and I. Herskowitz. 1998. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol. Cell 1:685-696. - PubMed
-
- Chu, S., J. DeRisi, M. Eisen, J. Mulholland, D. Botstein, P. O. Brown, and I. Herskowitz. 1998. The transcriptional program of sporulation in budding yeast. Science 282:699-705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
