Retinoic acid receptor alpha fusion to PML affects its transcriptional and chromatin-remodeling properties
- PMID: 14612419
- PMCID: PMC262687
- DOI: 10.1128/MCB.23.23.8795-8808.2003
Retinoic acid receptor alpha fusion to PML affects its transcriptional and chromatin-remodeling properties
Abstract
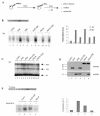
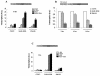
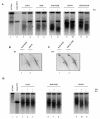
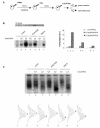
PML-RAR is an oncogenic transcription factor forming in acute promyelocytic leukemias (APL) because of a chromosomal translocation. Without its ligand, retinoic acid (RA), PML-RAR functions as a constitutive transcriptional repressor, abnormally associating with the corepressor-histone deacetylase complex and blocking hematopoietic differentiation. In the presence of pharmacological concentrations of RA, PML-RAR activates transcription and stimulates differentiation. Even though it has been suggested that chromatin alteration is important for APL onset, the PML-RAR effect on chromatin of target promoters has not been investigated. Taking advantage of the Xenopus oocyte system, we compared the wild-type transcription factor RARalpha with PML-RAR as both transcriptional regulators and chromatin structure modifiers. Without RA, we found that PML-RAR is a more potent transcriptional repressor that does not require the cofactor RXR and produces a closed chromatin configuration. Surprisingly, repression by PML-RAR occurs through a further pathway that is independent of nucleosome deposition and histone deacetylation. In the presence of RA, PML-RAR is a less efficient transcriptional activator that is unable to modify the DNA nucleoprotein structure. We propose that PML-RAR, aside from its ability to recruit aberrant quantities of histone deacetylase complexes, has acquired additional repressive mechanisms and lost important activating functions; the comprehension of these mechanisms might reveal novel targets for antileukemic intervention.
Figures


Similar articles
-
Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia.Blood. 1998 Apr 15;91(8):2634-42. Blood. 1998. PMID: 9531570
-
Growth suppression of acute promyelocytic leukemia cells having increased expression of the non-rearranged alleles: RAR alpha or PML.Oncogene. 1995 Jun 15;10(12):2307-14. Oncogene. 1995. PMID: 7784078
-
Characterization of the retinoid binding properties of the major fusion products present in acute promyelocytic leukemia cells.Blood. 1997 Aug 1;90(3):1175-85. Blood. 1997. PMID: 9242550
-
Characterisation of the PML/RAR alpha rearrangement associated with t(15;17) acute promyelocytic leukaemia.Curr Top Microbiol Immunol. 1997;220:81-112. doi: 10.1007/978-3-642-60479-9_6. Curr Top Microbiol Immunol. 1997. PMID: 9103677 Review.
-
The molecular biology of acute promyelocytic leukemia.Cancer Treat Res. 1999;99:75-124. doi: 10.1007/978-0-585-38571-6_4. Cancer Treat Res. 1999. PMID: 9891864 Review.
Cited by
-
The phenotypic reversion of cancer: Experimental evidences on cancer reversibility through epigenetic mechanisms (Review).Oncol Rep. 2024 Mar;51(3):48. doi: 10.3892/or.2024.8707. Epub 2024 Jan 26. Oncol Rep. 2024. PMID: 38275101 Free PMC article. Review.
-
Mechanism of histone H1-stimulated glucocorticoid receptor DNA binding in vivo.Mol Cell Biol. 2007 Mar;27(6):2398-410. doi: 10.1128/MCB.01509-06. Epub 2007 Jan 8. Mol Cell Biol. 2007. PMID: 17210632 Free PMC article.
-
Transposable Element Expression in Acute Myeloid Leukemia Transcriptome and Prognosis.Sci Rep. 2018 Nov 6;8(1):16449. doi: 10.1038/s41598-018-34189-x. Sci Rep. 2018. PMID: 30401833 Free PMC article.
-
Systems Biology for Drug Target Discovery in Acute Myeloid Leukemia.Int J Mol Sci. 2024 Apr 23;25(9):4618. doi: 10.3390/ijms25094618. Int J Mol Sci. 2024. PMID: 38731835 Free PMC article.
-
Interaction with RXR is necessary for NPM-RAR-induced myeloid differentiation blockade.Leuk Res. 2013 Dec;37(12):1704-10. doi: 10.1016/j.leukres.2013.09.024. Epub 2013 Sep 29. Leuk Res. 2013. PMID: 24183235 Free PMC article.
References
-
- Benedetti, L., A. A. Levin, B. M. Scicchitano, F. Grignani, G. Allanby, D. Diverio, F. Lo Coco, G. Avvisati, M. Ruthardt, S. Adamo, P. G. Pelicci, and C. Nervi. 1997. Characterization of the retinoid binding properties of the major fusion products present in acute promyelocytic leukemia cells. Blood 90:1175-1185. - PubMed
-
- Chen, H., R. J. Lin, W. Xie, D. Wilpitz, and R. M. Evans. 1999. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell 98:675-686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
