The mature activating natural killer cell immunologic synapse is formed in distinct stages
- PMID: 14612578
- PMCID: PMC283561
- DOI: 10.1073/pnas.1835830100
The mature activating natural killer cell immunologic synapse is formed in distinct stages
Abstract
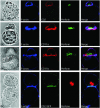
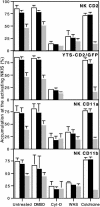
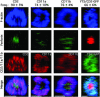
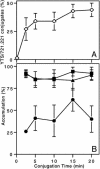
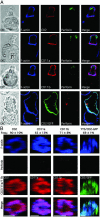
Natural killer (NK) cells form a structure at their interface with a susceptible target cell called the activating NK cell immunologic synapse (NKIS). The mature activating NKIS contains a central and peripheral supramolecular activation cluster (SMAC), and includes polarized surface receptors, filamentous actin (F-actin) and perforin. Evaluation of the NKIS in human NK cells revealed CD2, CD11a, CD11b and F-actin in the peripheral SMAC (pSMAC) with perforin in the central SMAC. The accumulation of F-actin and surface receptors was rapid and depended on Wiskott-Aldrich syndrome protein-driven actin polymerization. The accumulation at and arrangement of these molecules in the pSMAC was not affected by microtubule depolymerization. The polarization of perforin, however was slower and required intact actin, Wiskott-Aldrich syndrome protein, and microtubule function. Thus the process of CD2, CD11a, CD11b, and F-actin accumulation in the pSMAC and perforin accumulation in the central SMAC of the NKIS are sequential processes with distinct cytoskeletal requirements.
Figures

References
-
- Orange, J. S. (2002) Microbes Infect. 4, 1545–1558. - PubMed
-
- Miller, J. S. (2002) Cancer Invest. 20, 405–419. - PubMed
-
- Kohl, S., Springer, T. A., Schmalstieg, F. C., Loo, L. S. & Anderson, D. C. (1984) J. Immunol. 133, 2972–2978. - PubMed
-
- Bolhuis, R. L., Roozemond, R. C. & van de Griend, R. J. (1986) J. Immunol. 136, 3939–3944. - PubMed
-
- Inoue, H., Miyaji, M., Kosugi, A., Nagafuku, M., Okazaki, T., Mimori, T., Amakawa, R., Fukuhara, S., Domae, N., Bloom, E. T. & Umehara, H. (2002) Eur. J. Immunol. 32, 2188–2198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

