A phase II trial of capecitabine (Xeloda) in recurrent ovarian cancer
- PMID: 14612890
- PMCID: PMC2394434
- DOI: 10.1038/sj.bjc.6601381
A phase II trial of capecitabine (Xeloda) in recurrent ovarian cancer
Abstract
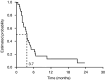
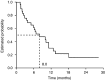
Oral capecitabine is a highly active, well-tolerated and convenient treatment for breast and colorectal cancer. This trial assessed the efficacy and safety of single-agent capecitabine in patients with previously treated ovarian cancer. A total of 29 patients with platinum-pretreated relapsed ovarian cancer were enrolled in this prospective, open-label, single-centre, phase II study. Patients received oral capecitabine 1250 mg m(-2) twice daily on days 1-14 of a 21-day cycle. Tumour response was evaluated using serum CA125. Out of 29 enrolled patients, 28 were evaluable, and a response was observed in eight patients (29%, 95% confidence interval (CI), 13-49%). Median progression-free and overall survivals were 3.7 (95% CI, 2.8-4.6) and 8.0 (95% CI, 4.1-11.8) months, respectively. After 6 months of treatment, 28% (95% CI, 13-48%) of patients remained progression-free and 62% (95% CI, 42-79%) were still alive. The most common clinical adverse events were hand-foot syndrome (HFS), nausea and diarrhoea. Grade 3 HFS occurred in 14% of patients, grade 3 vomiting in 10%. Efficacy and safety of capecitabine compare favourably with other monotherapies in platinum-refractory epithelial ovarian cancer. The convenience and improved safety profile of capecitabine compared with intravenous. regimens make it an ideal agent for administration in the outpatient setting.
Figures
References
-
- Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, Osterwalder B (2001) Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 92: 1759–1768 - PubMed
-
- Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T (1999) Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17: 485–493 - PubMed
-
- Boehmer CH, Jaeger W (2002) Capecitabine in treatment of platinum-resistant recurrent ovarian cancer. Anticancer Res 22: 439–444 - PubMed
-
- Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, Sulkes A, Greim G, Peters GJ, van der Born K, Wanders J, de Boer RF, Martin C, Fumoleau P (2002) Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer 38: 349–358 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous