Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity
- PMID: 14612912
- PMCID: PMC2394461
- DOI: 10.1038/sj.bjc.6601370
Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity
Abstract
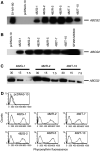
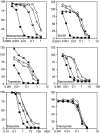
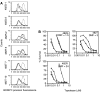
Recent studies have shown that mutations at amino-acid 482 in the ABCG2 gene affect the substrate specificity of the protein. To delineate the effects of these mutations clearly, human embryonic kidney cells (HEK-293) were stably transfected with wild-type 482R or mutant 482G and 482T ABCG2. By flow cytometry, mitoxantrone, BODIPY-prazosin, and Hoechst 33342 were found to be substrates of all ABCG2 proteins, while rhodamine 123, daunorubicin, and LysoTracker Green were transported only by mutant ABCG2. In cytotoxicity assays, all ABCG2 proteins conferred high levels of resistance to mitoxantrone, SN-38, and topotecan, while mutant ABCG2 also exhibited a gain of function for mitoxantrone as they conferred a four-fold greater resistance compared to wild type. Cells transfected with mutant ABCG2 were 13- to 71- fold resistant to the P-glycoprotein substrates doxorubicin, daunorubicin, epirubicin, bisantrene, and rhodamine 123 compared to cells transfected with wild-type ABCG2, which were only three- to four-fold resistant to these compounds. ABCG2 did not confer appreciable resistance to etoposide, taxol or the histone deacetylase inhibitor depsipeptide. None of the transfected cell lines demonstrated resistance to flavopiridol despite our previous observation that ABCG2-overexpressing cell lines are cross-resistant to the drug. Recently reported inhibitors of ABCG2 were evaluated and 50 microM novobiocin was found to reverse wild-type ABCG2 completely, but only reverse mutant ABCG2 partially. The studies presented here serve to underscore the importance of amino-acid 482 in defining the substrate specificity of the ABCG2 protein and raise the possibility that amino-acid 482 mutations in human cancers could affect the clinical application of antagonists for ABCG2.
Figures

Similar articles
-
Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells.Clin Cancer Res. 2001 Jan;7(1):145-52. Clin Cancer Res. 2001. PMID: 11205902
-
The role of breast cancer resistance protein in acute lymphoblastic leukemia.Clin Cancer Res. 2003 Nov 1;9(14):5171-7. Clin Cancer Res. 2003. PMID: 14613996
-
Single nucleotide polymorphisms modify the transporter activity of ABCG2.Cancer Chemother Pharmacol. 2005 Aug;56(2):161-72. doi: 10.1007/s00280-004-0931-x. Epub 2005 Apr 19. Cancer Chemother Pharmacol. 2005. PMID: 15838659
-
Flow cytometry-based approach to ABCG2 function suggests that the transporter differentially handles the influx and efflux of drugs.Cytometry A. 2004 Dec;62(2):129-38. doi: 10.1002/cyto.a.20072. Cytometry A. 2004. PMID: 15517563
-
Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2).Oncogene. 2003 Oct 20;22(47):7340-58. doi: 10.1038/sj.onc.1206938. Oncogene. 2003. PMID: 14576842 Review.
Cited by
-
Picky ABCG5/G8 and promiscuous ABCG2 - a tale of fatty diets and drug toxicity.FEBS Lett. 2020 Dec;594(23):4035-4058. doi: 10.1002/1873-3468.13938. Epub 2020 Oct 14. FEBS Lett. 2020. PMID: 32978801 Free PMC article. Review.
-
IMP3 protein promotes chemoresistance in breast cancer cells by regulating breast cancer resistance protein (ABCG2) expression.J Biol Chem. 2013 May 3;288(18):12569-73. doi: 10.1074/jbc.C112.442319. Epub 2013 Mar 28. J Biol Chem. 2013. PMID: 23539627 Free PMC article.
-
Methyl-Cantharidimide (MCA) Has Anticancer Efficacy in ABCB1- and ABCG2-Overexpressing and Cisplatin Resistant Cancer Cells.Front Oncol. 2020 Jun 26;10:932. doi: 10.3389/fonc.2020.00932. eCollection 2020. Front Oncol. 2020. PMID: 32676451 Free PMC article.
-
Reversal effect of FW-04-806, a macrolide dilactone compound, on multidrug resistance mediated by ABCB1 and ABCG2 in vitro and in vivo.Cell Commun Signal. 2019 Sep 1;17(1):110. doi: 10.1186/s12964-019-0408-5. Cell Commun Signal. 2019. PMID: 31472682 Free PMC article.
-
Interaction with the 5D3 monoclonal antibody is regulated by intramolecular rearrangements but not by covalent dimer formation of the human ABCG2 multidrug transporter.J Biol Chem. 2008 Sep 19;283(38):26059-70. doi: 10.1074/jbc.M803230200. Epub 2008 Jul 21. J Biol Chem. 2008. PMID: 18644784 Free PMC article.
References
-
- Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH (1999) The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 59: 4237–4241 - PubMed
-
- Allen JD, Jackson SC, Schinkel AH (2002) A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for doxorubicin resistance. Cancer Res 62: 2294–2299 - PubMed
-
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M (1998) A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58: 5337–5339 - PubMed
-
- Brangi M, Litman T, Ciotti M, Nishiyama K, Kohlhagen G, Takimoto C, Robey R, Pommier Y, Fojo T, Bates SE (1999) Camptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res 59: 5938–5946 - PubMed
-
- Chen G, Duran GE, Steger KA, Lacayo NJ, Jaffrezou JP, Dumontet C, Sikic BI (1997) Multidrug-resistant human sarcoma cells with a mutant P-glycoprotein altered phenotype and resistance to cyclosporins. J Biol Chem 272: 5974–5982 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

