Chitosan IFN-gamma-pDNA Nanoparticle (CIN) Therapy for Allergic Asthma
- PMID: 14613519
- PMCID: PMC280670
- DOI: 10.1186/1479-0556-1-3
Chitosan IFN-gamma-pDNA Nanoparticle (CIN) Therapy for Allergic Asthma
Abstract
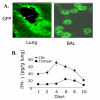
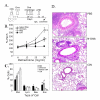
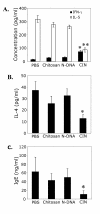
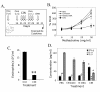
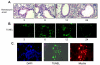
BACKGROUND: Allergic subjects produce relatively low amounts of IFN-gamma, a pleiotropic Th-1 cytokine that downregulates Th2-associated airway inflammation and hyperresponsiveness (AHR), the hallmarks of allergic asthma. Adenovirus-mediated IFN-gamma gene transfer reduces AHR, Th2 cytokine levels and lung inflammation in mice, but its use would be limited by the frequency of gene delivery required; therefore, we tested chitosan/IFN-gamma pDNA nanoparticles (CIN) for in situ production of IFN-gamma and its in vivo effects. METHODS: CIN were administered to OVA-sensitized mice to investigate the possibility of using gene transfer to modulate ovalbumin (OVA)-induced inflammation and AHR. RESULTS: Mice treated with CIN exhibit significantly lower AHR to methacholine challenge and less lung histopathology. Production of IFN-gamma is increased after CIN treatment while the Th2-cytokines, IL-4 and IL-5, and OVA-specific serum IgE are reduced compared to control mice. AHR and eosinophilia are also significantly reduced by CIN therapy administered therapeutically in mice with established asthma. CIN was found to inhibit epithelial inflammation within 6 hours of delivery by inducing apoptosis of goblet cells. Experiments performed on STAT4-defective mice do not show reduction in AHR with CIN treatment, thus implicating STAT4 signaling in the mechanism of CIN action. CONCLUSION: These results demonstrate that mucosal CIN therapy can effectively reduce established allergen-induced airway inflammation and AHR.
Figures

References
-
- Umetsu DT, Dekruyff RH. TH1 and TH2 CD4+ cells in human allergic diseases. J Allergy Clin Immunol. 1997;100:1–6. - PubMed
-
- Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, Kurup VP, Warnock M, Grunig G. IL-13 and IFN-gamma: interactions in lung inflammation. J Immunol. 2001;167:1769–1777. - PubMed
-
- Daines MO, Khurana Hershey GK. A novel mechanism by which interferon-gamma can regulate IL-13 responses: Evidence for intracellular stores of IL-13 receptor alpha 2 and their rapid mobilization by interferon-gamma. J Biol Chem. 2002. [epub ahead of print]. - PubMed
-
- Boraschi D, Censini S, Bartalini M, Ghiara P, Di Simplicio P, Tagliabue A. Interferons inhibit LTC4 production in murine macrophages. J Immunol. 1987;138:4341–4346. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

