Role of tetrodotoxin-resistant Na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons
- PMID: 14614093
- PMCID: PMC6741008
- DOI: 10.1523/JNEUROSCI.23-32-10338.2003
Role of tetrodotoxin-resistant Na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons
Abstract
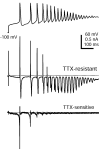
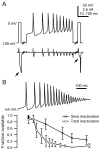
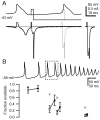
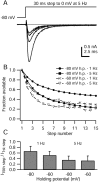
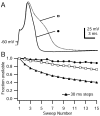
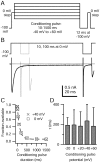
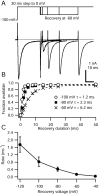
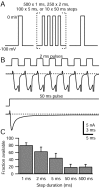
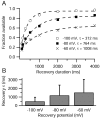
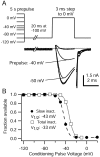
When acutely dissociated small-diameter dorsal root ganglion (DRG) neurons were stimulated with repeated current injections or prolonged application of capsaicin, their action potential firing quickly adapted. Because TTX-resistant (TTX-R) sodium current in these presumptive nociceptors generates a large fraction of depolarizing current during the action potential, we examined the possible role of inactivation of TTX-R sodium channels in producing adaptation. Under voltage clamp, TTX-R current elicited by short depolarizations showed strong use dependence at frequencies as low as 1 Hz, although recovery from fast inactivation was complete in approximately 10-30 msec. This use-dependent reduction was the result of the entry of TTX-R sodium channels into slow inactivated states. Slow inactivation was more effectively produced by steady depolarization than by cycling channels through open states. Slow inactivation was steeply voltage dependent, with a Boltzmann slope factor of 5 mV, a midpoint near -45 mV (5 sec conditioning pulses), and completeness of approximately 93% positive to -20 mV. The time constant for entry (approximately 200 msec) was independent of voltage from -20 mV to +60 mV, whereas recovery kinetics were moderately voltage dependent (time constant, approximately 1.5 sec at -60 mV and approximately 0.5 sec at -100 mV). Using a prerecorded current-clamp response to capsaicin as a voltage-clamp command waveform, we found that adaptation of firing occurred with a time course similar to that of development of slow inactivation. Thus, slow inactivation of the TTX-R sodium current limits the duration of small DRG cell firing in response to maintained stimuli and may contribute to cross desensitization between chemical and electrical stimuli.
Figures

References
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN (1999) The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2:541-548. - PubMed
-
- Andrew D, Greenspan JD (1999) Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol 82:2649-2656. - PubMed
-
- Baker MD, Wood JN (2001) Involvement of Na+ channels in pain pathways. Trends Pharmacol Sci 22:27-31. - PubMed
-
- Baumann TK, Burchiel KJ, Ingram SL, Martenson ME (1996) Responses of adult human dorsal root ganglion neurons in culture to capsaicin and low pH. Pain 65:31-38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
