Electrophysiological differentiation of new neurons in the olfactory bulb
- PMID: 14614100
- PMCID: PMC6741027
- DOI: 10.1523/JNEUROSCI.23-32-10411.2003
Electrophysiological differentiation of new neurons in the olfactory bulb
Abstract
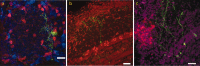
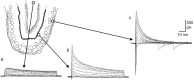
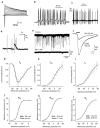
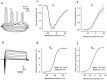
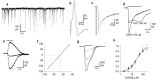
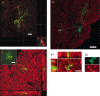
The subventricular zone produces neuroblasts that migrate to the olfactory bulb (OB) and differentiate into interneurons throughout postnatal life (Altman and Das, 1966; Hinds, 1968; Altman, 1969; Kishi et al., 1990; Luskin, 1993; Lois and Alvarez-Buylla, 1994). Although such postnatally generated interneurons have been characterized morphologically, their physiological differentiation has not been thoroughly described. Combining retroviral-mediated labeling of newly generated neurons with patch-clamp electrophysiology, we demonstrated that soon after new cells enter the layers of the olfactory bulb, they display voltage-dependent currents typical of more mature neurons. We also show that these "newcomers" express functional GABA and glutamate receptor channels, respond synaptically to stimulation of the olfactory nerve, and may establish both axodendritic and dendrodendritic synaptic contacts within the olfactory bulb. These data provide a basic description of the physiology of newly generated cells in the OB and show that such new cells are functional neurons that synaptically integrate into olfactory bulb circuitry soon after their arrival.
Figures

References
-
- Altman J ( 1969) Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433-458. - PubMed
-
- Altman J, Das GD ( 1966) Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol 126: 337-389. - PubMed
-
- Alvarez-Buylla A, Temple S ( 1998) Stem cells in the developing and adult nervous system. J Neurobiol 36: 105-110. - PubMed
-
- Baker H, Towle AC, Margolis FL ( 1988) Differential afferent regulation of dopaminergic and GABAergic neurons in the mouse main olfactory bulb. Brain Res 450: 69-80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
