Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses
- PMID: 14614102
- PMCID: PMC6741001
- DOI: 10.1523/JNEUROSCI.23-32-10433.2003
Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses
Abstract
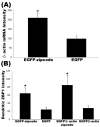
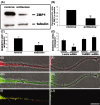
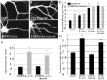
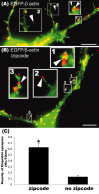
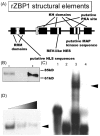
The dendritic transport and local translation of mRNA may be an essential mechanism to regulate synaptic growth and plasticity. We investigated the molecular mechanism and function of beta-actin mRNA localization in dendrites of cultured hippocampal neurons. Previous studies have shown that beta-actin mRNA localization to the leading edge of fibroblasts or the growth cones of developing neurites involved a specific interaction between a zipcode sequence in the 3' untranslated region and the mRNA-binding protein zipcode-binding protein-1 (ZBP1). Here, we show that ZBP1 is required for the localization of beta-actin mRNA to dendrites. Knock-down of ZBP1 using morpholino antisense oligonucleotides reduced dendritic levels of ZBP1 and beta-actin mRNA and impaired growth of dendritic filopodia in response to BDNF treatment. Transfection of an enhanced green fluorescent protein (EGFP)-beta-actin construct, which contained the zipcode, increased the density of dendritic filopodia and filopodial synapses. Transfection of an EGFP construct, also with the zipcode, resulted in recruitment of endogenous ZBP1 and beta-actin mRNA into dendrites and similarly increased the density of dendritic filopodia. However, the beta-actin zipcode did not affect filopodial length or the density of mature spines. These results reveal a novel function for an mRNA localization element and its binding protein in the regulation of dendritic morphology and synaptic growth via dendritic filopodia.
Figures



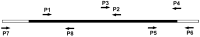
References
-
- Bassell GJ, Oleynikov Y, Singer RH ( 1999) The travels of mRNAs through all cells large and small. FASEB J 13: 447-454. - PubMed
-
- Blichenberg A, Rehbein M, Muller R, Garner CC, Richter D, Kindler S ( 2001) Identification of a cis-acting dendritic targeting element in the mRNA encoding the alpha subunit of CaMKII. Eur J Neurosci 13: 1-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
