Conversion of the allosteric transition of GroEL from concerted to sequential by the single mutation Asp-155 -> Ala
- PMID: 14615587
- PMCID: PMC283501
- DOI: 10.1073/pnas.2333925100
Conversion of the allosteric transition of GroEL from concerted to sequential by the single mutation Asp-155 -> Ala
Abstract
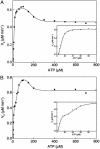
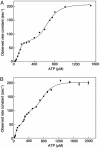
The reaction cycle of the double-ring chaperonin GroEL is driven by ATP binding that takes place with positive cooperativity within each seven-membered ring and negative cooperativity between rings. The positive cooperativity within rings is due to ATP binding-induced conformational changes that are fully concerted. Herein, it is shown that the mutation Asp-155 --> Ala leads to an ATP-induced break in intra-ring and inter-ring symmetry. Electron microscopy analysis of single-ring GroEL particles containing the Asp-155 --> Ala mutation shows that the break in intra-ring symmetry is due to stabilization of allosteric intermediates such as one in which three subunits have switched their conformation while the other four have not. Our results show that eliminating an intra-subunit interaction between Asp-155 and Arg-395 results in conversion of the allosteric switch of GroEL from concerted to sequential, thus demonstrating that its allosteric behavior arises from coupled tertiary conformational changes.
Figures
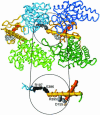

Similar articles
-
A kinetic analysis of the nucleotide-induced allosteric transitions in a single-ring mutant of GroEL.J Mol Biol. 2004 May 14;338(5):969-77. doi: 10.1016/j.jmb.2004.03.010. J Mol Biol. 2004. PMID: 15111060
-
Kinetic analysis of ATP-dependent inter-ring communication in GroEL.J Mol Biol. 2004 May 14;338(5):979-88. doi: 10.1016/j.jmb.2004.02.076. J Mol Biol. 2004. PMID: 15111061
-
Mapping pathways of allosteric communication in GroEL by analysis of correlated mutations.Proteins. 2002 Sep 1;48(4):611-7. doi: 10.1002/prot.10180. Proteins. 2002. PMID: 12211028
-
Structure and allostery of the chaperonin GroEL.J Mol Biol. 2013 May 13;425(9):1476-87. doi: 10.1016/j.jmb.2012.11.028. Epub 2012 Nov 24. J Mol Biol. 2013. PMID: 23183375 Review.
-
Review: allostery in chaperonins.J Struct Biol. 2001 Aug;135(2):104-14. doi: 10.1006/jsbi.2001.4377. J Struct Biol. 2001. PMID: 11580260 Review.
Cited by
-
Allostery and cooperativity revisited.Protein Sci. 2008 Aug;17(8):1295-307. doi: 10.1110/ps.03259908. Epub 2008 Jun 17. Protein Sci. 2008. PMID: 18560010 Free PMC article. Review.
-
Concerted ATP-induced allosteric transitions in GroEL facilitate release of protein substrate domains in an all-or-none manner.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3119-24. doi: 10.1073/pnas.0700070104. Epub 2007 Feb 21. Proc Natl Acad Sci U S A. 2007. PMID: 17360617 Free PMC article.
-
Football- and bullet-shaped GroEL-GroES complexes coexist during the reaction cycle.J Biol Chem. 2008 Aug 29;283(35):23765-73. doi: 10.1074/jbc.M802541200. Epub 2008 Jun 20. J Biol Chem. 2008. PMID: 18567585 Free PMC article.
-
Classification of Single Particles from Human Cell Extract Reveals Distinct Structures.Cell Rep. 2018 Jul 3;24(1):259-268.e3. doi: 10.1016/j.celrep.2018.06.022. Cell Rep. 2018. PMID: 29972786 Free PMC article.
-
Crystal structure of a GroEL-ADP complex in the relaxed allosteric state at 2.7 Å resolution.Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):E2958-66. doi: 10.1073/pnas.1311996110. Epub 2013 Jul 16. Proc Natl Acad Sci U S A. 2013. PMID: 23861496 Free PMC article.
References
-
- Perutz, M. F. (1989) Q. Rev. Biophys. 22, 139–237. - PubMed
-
- Monod, J., Wyman, J. & Changeux, J.–P. (1965) J. Mol. Biol. 12, 88–118. - PubMed
-
- Koshland, D. E. Jr., Némethy, G. & Filmer, D. (1966) Biochemistry 5, 365–385. - PubMed
-
- Eigen, M. (1967) Nobel Symp. 5, 333–369.
-
- Thirumalai, D. & Lorimer, G. H. (2001) Annu. Rev. Biophys. Biomol. Struct. 30, 245–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

