Ethylene regulates arabidopsis development via the modulation of DELLA protein growth repressor function
- PMID: 14615596
- PMCID: PMC282807
- DOI: 10.1105/tpc.015685
Ethylene regulates arabidopsis development via the modulation of DELLA protein growth repressor function
Abstract
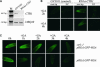
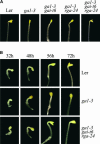
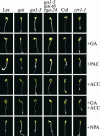
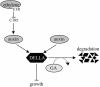
Phytohormones regulate plant development via a poorly understood signal response network. Here, we show that the phytohormone ethylene regulates plant development at least in part via alteration of the properties of DELLA protein nuclear growth repressors, a family of proteins first identified as gibberellin (GA) signaling components. This conclusion is based on the following experimental observations. First, ethylene inhibited Arabidopsis root growth in a DELLA-dependent manner. Second, ethylene delayed the GA-induced disappearance of the DELLA protein repressor of ga1-3 from root cell nuclei via a constitutive triple response-dependent signaling pathway. Third, the ethylene-promoted "apical hook" structure of etiolated seedling hypocotyls was dependent on the relief of DELLA-mediated growth restraint. Ethylene, auxin, and GA responses now can be attributed to effects on DELLA function, suggesting that DELLA plays a key integrative role in the phytohormone signal response network.
Figures



References
-
- Abeles, F.B., Morgan, P.W., and Saltveit, M.E., Jr. (1992). Ethylene in Plant Biology. (San Diego, CA: Academic Press).
-
- Bouquin, T., Mattsson, O., Naested, H., Foster, R., and Mundy, J. (2002). The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J. Cell Sci. 116, 791–801. - PubMed
-
- Celenza, J.L., Grisafi, P.L., and Fink, G.R. (1995). A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9, 2131–2142. - PubMed
-
- Chen, Y.F., Randlett, M.D., Findell, J.L., and Schaller, G.E. (2002). Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J. Biol. Chem. 277, 19861–19866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

