Tapetum determinant1 is required for cell specialization in the Arabidopsis anther
- PMID: 14615601
- PMCID: PMC282801
- DOI: 10.1105/tpc.016618
Tapetum determinant1 is required for cell specialization in the Arabidopsis anther
Abstract
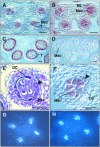
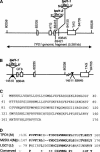
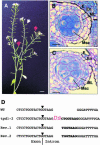
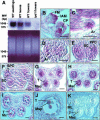
In flowering plants, pollen formation depends on the differentiation and interaction of two cell types in the anther: the reproductive cells, called microsporocytes, and somatic cells that form the tapetum. The microsporocytes generate microspores, whereas the tapetal cells support the development of microspores into mature pollen grains. Despite their importance to plant reproduction, little is known about the underlying genetic mechanisms that regulate the differentiation and interaction of these highly specialized cells in the anther. Here, we report the identification and characterization of a novel tapetum determinant1 (TPD1) gene that is required for the specialization of tapetal cells in the Arabidopsis anther. Analysis of the male-sterile mutant, tpd1, showed that functional interruption of TPD1 caused the precursors of tapetal cells to differentiate and develop into microsporocytes instead of tapetum. As a results, extra microsporocytes were formed and tapetum was absent in developing tpd1 anthers. Molecular cloning of TPD1 revealed that it encodes a small protein of 176 amino acids. In addition, tpd1 was phenotypically similar to excess microsporocytes1/extra sporogenous cells (ems1/exs) single and tpd1 ems1/exs double mutants. These data suggest that the TPD1 product plays an important role in the differentiation of tapetal cells, possibly in coordination with the EMS1/EXS gene product, a Leu-rich repeat receptor protein kinase.
Figures


References
-
- Canales, C., Bhatt, A.M., Scott, R., and Dickinson, H. (2002). EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr. Biol. 12, 1718–1727. - PubMed
-
- Dawson, J., Wilson, Z.A., Aarts, M.G.M., Braithwaite, A.F., Briarty, L.G., and Mulligan, N.J. (1993). Microspore and pollen development in six male-sterile mutants of Arabidopsis thaliana. Can. J. Bot. 71, 629–638.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

