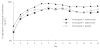No clinically significant effect of erythromycin or azithromycin on the pharmacokinetics of voriconazole in healthy male volunteers
- PMID: 14616411
- PMCID: PMC1884310
- DOI: 10.1046/j.1365-2125.2003.01996.x
No clinically significant effect of erythromycin or azithromycin on the pharmacokinetics of voriconazole in healthy male volunteers
Abstract
Aims: The antibiotic erythromycin is a potent inhibitor of cytochrome P450 CYP3A4 metabolism. As CYP isozymes, including CYP3A4, are involved in the metabolism of the new triazole voriconazole, this study investigated the effects of multiple-dose erythromycin or azithromycin on the steady-state pharmacokinetics of voriconazole in healthy male subjects.
Methods: In an open, randomized, parallel-group, single-centre study, 30 healthy male subjects aged 20-41 years received oral voriconazole 200 mg twice daily for 14 days plus either erythromycin (1 g twice daily on days 8-14), azithromycin (500 mg once daily on days 12-14) or placebo (twice daily on days 8-14). Only morning doses were administered on day 14. Plasma concentrations of voriconazole were measured up to 12 h postdose on days 7 and 14, and plasma pharmacokinetic parameters were calculated. Adverse events and standard laboratory test results were recorded before and throughout the study.
Results: Comparison of the voriconazole Cmax day 14/day 7 ratio for the voriconazole + erythromycin group with that of the voriconazole + placebo group yielded a ratio of 107.7%[90% confidence interval (CI) 90.6, 128.0]; for the voriconazole + azithromycin group, the ratio was 117.5% (90% CI 98.8, 139.7). Comparison of the voriconazole AUCtau day 14/day 7 ratios of the voriconazole + erythromycin and voriconazole + azithromycin groups with that of the voriconazole + placebo group showed ratios of 101.2% (90% CI 89.1, 114.8) and 107.9% (90% CI 95.1, 122.4), respectively. For voriconazole tmax, the differences between the day 14-day 7 calculations for the voriconazole + erythromycin or the voriconazole + azithromycin groups and that of the voriconazole + placebo group were - 0.2 h (90% CI - 0.8, 0.3) and - 0.1 h (90% CI - 0.7, 0.5), respectively. None of these changes was considered clinically relevant. The study drugs were well tolerated by subjects in all groups; the most common study drug-related adverse events were visual disturbances, reported in all groups, and abdominal pain, present in the voriconazole + erythromycin group.
Conclusions: Coadministration of erythromycin or azithromycin does not affect the steady-state pharmacokinetics of voriconazole in a clinically relevant manner in healthy male subjects.
Figures
References
-
- Cuenca-Estrella M, Rodriguez-Tudela JL, Mellado E, Martinez-Suarez JV, Monzon A. Comparison of the in vitro activity of voriconazole (UK-109,496), itraconazole and amphotericin B against clinical isolates of Aspergillus fumigatus. J Antimicrob Chemother. 1998;42:531–533. - PubMed
-
- Chandrasekar PH, Manavathu E. Voriconazole: a second-generation triazole. Drugs Today. 2001;37:135–148. - PubMed
-
- Torre-Cisneros J, Gonzalez-Ruiz A, Hodges MR, Lutsar I. Program and Abstracts of the 38th Annual Meeting of the Infectious Diseases Society of America. New Orleans, LA; 2000. Voriconazole (VORI) for the treatment of S. apiospermum and S. prolificans infection. Abstract 305, September.
-
- Perfect J, Gonzalez-Ruiz A, Lutsar I. Program and Abstracts of the 38th Annual Meeting of the Infectious Diseases Society of America. New Orleans, LA; 2000. Voriconazole (VORI) for the treatment of resistant and rare fungal pathogens. Abstract 303, September.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials