The application of biofilm science to the study and control of chronic bacterial infections
- PMID: 14617746
- PMCID: PMC259139
- DOI: 10.1172/JCI20365
The application of biofilm science to the study and control of chronic bacterial infections
Erratum in
- J Clin Invest. 2007 Jan;117(1):278
Abstract
Unequivocal direct observations have established that the bacteria that cause device-related and other chronic infections grow in matrix-enclosed biofilms. The diagnostic and therapeutic strategies that have served us so well in the partial eradication of acute epidemic bacterial diseases have not yielded accurate data or favorable outcomes when applied to these biofilm diseases. We discuss the potential benefits of the application of the new methods and concepts developed by biofilm science and engineering to the clinical management of infectious diseases.
Figures
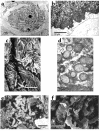




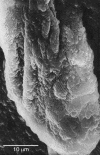
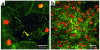

References
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. - PubMed
-
- Marrie TJ, Nelligan J, Costerton JW. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982;66:1339–1341. - PubMed
-
- Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO Transactions. 1992;38:M174–M178. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

