Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency
- PMID: 14617756
- PMCID: PMC259128
- DOI: 10.1172/JCI18784
Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency
Abstract
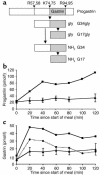
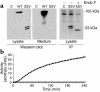
We have previously described the only reported case of human proprotein convertase 1 (PC1) deficiency, in a female (Subject A) with obesity, hypogonadism, hypoadrenalism, and reactive hypoglycemia. We now report the second case of human PC1 deficiency (Subject B), also due to compound heterozygosity for novel missense and nonsense mutations. While both subjects shared the phenotypes of obesity, hypoadrenalism, reactive hypoglycemia, and elevated circulating levels of certain prohormones, the clinical presentation of Subject B was dominated by severe refractory neonatal diarrhea, malabsorptive in type. Subsequent investigation of Subject A revealed marked small-intestinal absorptive dysfunction, which was not previously clinically suspected. We postulate that PC1, presumably in the enteroendocrine cells, is essential for the normal absorptive function of the human small intestine. The differences in the nature and severity of presentation between the two cases cannot readily be explained on the basis of allelic heterogeneity, as the nonsense and missense mutations from both subjects had comparably severe effects on the catalytic activity of PC1. Despite Subject A's negligible PC1 activity, some mature ACTH and glucagon-like peptide 17-36(amide) were detectable in her plasma, suggesting that the production of these hormones, at least in humans, does not have an absolute dependence on PC1. The presence of severe obesity and the absence of growth retardation in both subjects contrast markedly with the phenotype of mice lacking PC1 and suggest that the precise physiological repertoire of this enzyme may vary between mammalian species.
Figures


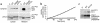

References
-
- Wilson HE, White A. Prohormones: their clinical relevance. Trends Endocrinol. Metab. 1998;9:396–402. - PubMed
-
- Steiner D. The proprotein convertases. Curr. Opin. Chem. Biol. 1998;2:31–39. - PubMed
-
- Rouille Y, et al. Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front. Neuroendocrinol. 1995;16:322–361. - PubMed
-
- Bertagna X. Proopiomelanocortin-derived peptides. Endocrinol. Metab. Clin. North Am. 1994;23:467–485. - PubMed
-
- Dhanvantari S, Seidah NG, Brubaker PL. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol. Endocrinol. 1996;10:342–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

