Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice
- PMID: 14617773
- PMCID: PMC283567
- DOI: 10.1073/pnas.2332818100
Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice
Abstract
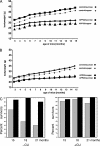
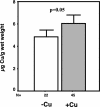
The Cu-binding beta-amyloid precursor protein (APP), and the amyloid Abeta peptide have been proposed to play a role in physiological metal regulation. There is accumulating evidence of an unbalanced Cu homeostasis with a causative or diagnostic link to Alzheimer's disease. Whereas elevated Cu levels are observed in APP knockout mice, APP overexpression results in reduced Cu in transgenic mouse brain. Moreover, Cu induces a decrease in Abeta levels in APP-transfected cells in vitro. To investigate the influence of bioavailable Cu, transgenic APP23 mice received an oral treatment with Cu-supplemented sucrose-sweetened drinking water (1). Chronic APP overexpression per se reduced superoxide dismutase 1 activity in transgenic mouse brain, which could be restored to normal levels after Cu treatment (2). A significant increase of brain Cu indicated its bioavailability on Cu treatment in APP23 mice, whereas Cu levels remained unaffected in littermate controls (3). Cu treatment lowered endogenous CNS Abeta before a detectable reduction of amyloid plaques. Thus, APP23 mice reveal APP-induced alterations linked to Cu homeostasis, which can be reversed by addition of dietary Cu.
Figures
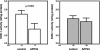

References
-
- Selkoe, D. J. (2001) Physiol. Rev. 81, 741–766. - PubMed
-
- Tanzi, R. E., Gusella, J. F., Watkins, P. C., Bruns, G. A., St. George-Hyslop, P., Van Keuren, M. L., Patterson, D., Pagan, S., Kurnit, D. M. & Neve, R. L. (1987) Science 235, 880–884. - PubMed
-
- Kang, J., Lemaire, H. G., Unterbeck, A., Salbaum, J. M., Masters, C. L., Grzeschik, K. H., Multhaup, G., Beyreuther, K. & Muller-Hill, B. (1987) Nature 325, 733–736. - PubMed
-
- Dodart, J. C., Mathis, C. & Ungerer, A. (2000) Rev. Neurosci. 11, 75–93. - PubMed
-
- Hesse, L., Beher, D., Masters, C. L. & Multhaup, G. (1994) FEBS Lett. 349, 109–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

