Transcriptional activation of a constitutive heterochromatic domain of the human genome in response to heat shock
- PMID: 14617804
- PMCID: PMC329232
- DOI: 10.1091/mbc.e03-07-0487
Transcriptional activation of a constitutive heterochromatic domain of the human genome in response to heat shock
Abstract
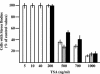
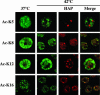
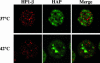
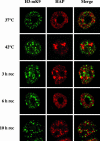
Heat shock triggers the assembly of nuclear stress bodies that contain heat shock factor 1 and a subset of RNA processing factors. These structures are formed on the pericentromeric heterochromatic regions of specific human chromosomes, among which chromosome 9. In this article we show that these heterochromatic domains are characterized by an epigenetic status typical of euchromatic regions. Similarly to transcriptionally competent portions of the genome, stress bodies are, in fact, enriched in acetylated histone H4. Acetylation peaks at 6 h of recovery from heat shock. Moreover, heterochromatin markers, such as HP1 and histone H3 methylated on lysine 9, are excluded from these nuclear districts. In addition, heat shock triggers the transient accumulation of RNA molecules, heterogeneous in size, containing the subclass of satellite III sequences found in the pericentromeric heterochromatin of chromosome 9. This is the first report of a transcriptional activation of a constitutive heterochromatic portion of the genome in response to stress stimuli.
Figures

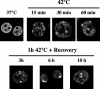
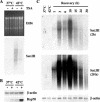

References
-
- Agalioti, T., Chen, G., and Thanos, D. (2002). Deciphering the transcriptional histone acetylation code for a human gene. Cell 111, 381-392. - PubMed
-
- Boumil, R.M., and Lee, J.T. (2001). Forty years of decoding the silence in X-chromosome inactivation. Hum. Mol. Genet. 10, 2225-2232. - PubMed
-
- Brockdorff, N. (2002). X-chromosome inactivation: closing in on proteins that bind Xist RNA. Trends Genet 18, 352-358. - PubMed
-
- Chiodi, I., Biggiogera, M., Denegri, M., Corioni, M., Weighardt, F., Cobianchi, F., Riva, S., and Biamonti, G. (2000). Structure and dynamics of hnRNP-labelled nuclear bodies induced by stress treatments. J. Cell Sci. 113, 4043-4053. - PubMed
-
- Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J., and Rutter, W.J. (1979). Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18, 5294-5299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

