Gelsolin mediates collagen phagocytosis through a rac-dependent step
- PMID: 14617805
- PMCID: PMC329256
- DOI: 10.1091/mbc.e03-07-0468
Gelsolin mediates collagen phagocytosis through a rac-dependent step
Abstract
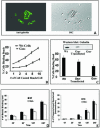
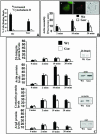
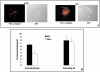
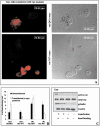
The role of gelsolin, a calcium-dependent actin-severing protein, in mediating collagen phagocytosis, is not defined. We examined alpha 2 beta 1 integrin-mediated phagocytosis in fibroblasts from wild-type (WT) and gelsolin knockout (Gsn(-)) mice. After initial contact with collagen beads, collagen binding and internalization were 60% lower in Gsn(-) than WT cells. This deficiency was restored by transfection with gelsolin or with beta1 integrin-activating antibodies. WT cells showed robust rac activation and increased [Ca(2+)](i) during early contact with collagen beads, but Gsn(-) cells showed very limited responses. Transfected gelsolin in Gsn(-) cells restored rac activation after collagen binding. Transfection of Gsn(-) cells with active rac increased collagen binding to WT levels. Chelation of intracellular calcium inhibited collagen binding and rac activation, whereas calcium ionophore induced rac activation in WT and Gsn(-) cells. We conclude that the ability of gelsolin to remodel actin filaments is important for collagen-induced calcium entry; calcium in turn is required for rac activation, which subsequently enhances collagen binding to unoccupied alpha 2 beta 1 integrins.
Figures



References
-
- Allen, P.G., and Janmey, P.A. (1994). Gelsolin displaces phalloidin from actin filaments. A new fluorescence method shows that both Ca2+ and Mg2+ affect the rate at which gelsolin severs F-actin. J. Biol. Chem. 269, 32916-32923. - PubMed
-
- Arcaro, A. (1998). The small GTP-binding protein rac promotes the dissociation of gelsolin from actin filaments in the neutrophils. J. Biol. Chem. 273, 805-813. - PubMed
-
- Arora, P.D., Bibby, K.J., and McCulloch, C.A. (1994). Slow oscillations of free intracellular calcium ion concentration in human fibroblasts responding to mechanical stretch. J. Cell. Physiol. 161, 187-200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

