Repositioning of muscle-specific genes relative to the periphery of SC-35 domains during skeletal myogenesis
- PMID: 14617810
- PMCID: PMC307540
- DOI: 10.1091/mbc.e03-06-0388
Repositioning of muscle-specific genes relative to the periphery of SC-35 domains during skeletal myogenesis
Abstract
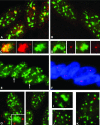
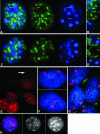
Previous studies have shown that in a given cell type, certain active genes associate with SC-35 domains, nuclear regions rich in RNA metabolic factors and excluded from heterochromatin. This organization is not seen for all active genes; therefore, it is important to determine whether and when this locus-specific organization arises during development and differentiation of specific cell types. Here, we investigate whether gene organization relative to SC-35 domains is cell type specific by following several muscle and nonmuscle genes in human fibroblasts, committed but proliferative myoblasts, and terminally differentiated muscle. Although no change was seen for other loci, two muscle genes (Human beta-cardiac myosin heavy chain and myogenin) became localized to the periphery of an SC-35 domain in terminally differentiated muscle nuclei, but not in proliferative myoblasts or in fibroblasts. There was no apparent change in gene localization relative to either the chromosome territory or the heterochromatic compartment; thus, the gene repositioning seemed to occur specifically with respect to SC-35 domains. This gene relocation adjacent to a prominent SC-35 domain was recapitulated in mouse 3T3 cells induced into myogenesis by introduction of MyoD. Results demonstrate a cell type-specific reorganization of specific developmentally regulated loci relative to large domains of RNA metabolic factors, which may facilitate developmental regulation of genome expression.
Figures
Similar articles
-
MyoD and myogenin expression patterns in cultures of fetal and adult chicken myoblasts.J Histochem Cytochem. 2001 Apr;49(4):455-62. doi: 10.1177/002215540104900405. J Histochem Cytochem. 2001. PMID: 11259448
-
MUNC, a long noncoding RNA that facilitates the function of MyoD in skeletal myogenesis.Mol Cell Biol. 2015 Feb;35(3):498-513. doi: 10.1128/MCB.01079-14. Epub 2014 Nov 17. Mol Cell Biol. 2015. PMID: 25403490 Free PMC article.
-
MyoD Overexpressed Equine Adipose-Derived Stem Cells Enhanced Myogenic Differentiation Potential.Cell Transplant. 2016 Nov;25(11):2017-2026. doi: 10.3727/096368916X691015. Cell Transplant. 2016. PMID: 26892394
-
Involvement of transportin 2-mediated HuR import in muscle cell differentiation.Mol Biol Cell. 2007 Jul;18(7):2619-29. doi: 10.1091/mbc.e07-02-0167. Epub 2007 May 2. Mol Biol Cell. 2007. PMID: 17475777 Free PMC article.
-
Dual promoter structure of ZFP106: regulation by myogenin and nuclear respiratory factor-1.Gene. 2005 Jan 3;344:143-59. doi: 10.1016/j.gene.2004.09.035. Epub 2004 Nov 19. Gene. 2005. PMID: 15656981
Cited by
-
Gene expression, chromosome position and lamin A/C mutations.Nucleus. 2011 May-Jun;2(3):162-7. doi: 10.4161/nucl.2.3.16003. Nucleus. 2011. PMID: 21818408 Free PMC article.
-
Visualizing transcription factor dynamics in living cells.J Cell Biol. 2018 Apr 2;217(4):1181-1191. doi: 10.1083/jcb.201710038. Epub 2018 Jan 29. J Cell Biol. 2018. PMID: 29378780 Free PMC article. Review.
-
Association of adipogenic genes with SC-35 domains during porcine adipogenesis.Chromosome Res. 2010 Dec;18(8):887-95. doi: 10.1007/s10577-010-9176-1. Epub 2010 Dec 3. Chromosome Res. 2010. PMID: 21127962
-
Association between active genes occurs at nuclear speckles and is modulated by chromatin environment.J Cell Biol. 2008 Sep 22;182(6):1083-97. doi: 10.1083/jcb.200803174. J Cell Biol. 2008. PMID: 18809724 Free PMC article.
-
Clustering of multiple specific genes and gene-rich R-bands around SC-35 domains: evidence for local euchromatic neighborhoods.J Cell Biol. 2003 Sep 15;162(6):981-90. doi: 10.1083/jcb.200303131. J Cell Biol. 2003. PMID: 12975345 Free PMC article.
References
-
- Bickmore, W.A., and Sumner, A.T. (1989). Mammalian chromosome banding–an expression of genome organization. Trends Genet. 5, 144–148. - PubMed
-
- Braun, T., Winter, B., Bober, E., and Arnold, H.H. (1990). Transcriptional activation domain of the muscle-specific gene-regulatory protein myf5. Nature 346, 663–665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

