Rabip4' is an effector of rab5 and rab4 and regulates transport through early endosomes
- PMID: 14617813
- PMCID: PMC329268
- DOI: 10.1091/mbc.e03-05-0343
Rabip4' is an effector of rab5 and rab4 and regulates transport through early endosomes
Abstract
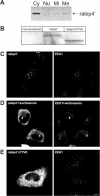
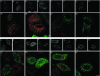
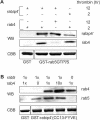
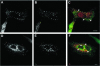
We describe the characterization of an 80-kDa protein cross-reacting with a monoclonal antibody against the human La autoantigen. The 80-kDa protein is a variant of rabip4 with an N-terminal extension of 108 amino acids and is expressed in the same cells. For this reason, we named it rabip4'. rabip4' is a peripheral membrane protein, which colocalized with internalized transferrin and EEA1 on early endosomes. Membrane association required the presence of the FYVE domain and was perturbed by the phosphatidylinositol 3-kinase inhibitor wortmannin. Expression of a dominant negative rabip4' mutant reduced internalization and recycling of transferrin from early endosomes, suggesting that it may be functionally linked to rab4 and rab5. In agreement with this, we found that rabip4' colocalized with the two GTPases on early endosomes and bound specifically and simultaneously to the GTP form of both rab4 and rab5. We conclude that rabip4' may coordinate the activities of rab4 and rab5, regulating membrane dynamics in the early endosomal system.
Figures





References
-
- Alber, T. (1992). Structure of the leucine zipper. Curr. Opin. Genet. Dev. 2, 205-210. - PubMed
-
- Bottger, G., Nagelkerken, B., and van der Sluijs, P. (1996). Rab4 and rab7 define distinct endocytic compartments. J. Biol. Chem. 271, 29191-29197. - PubMed
-
- Bucci, C., Parton, R., Mather, I., Stunnenberg, H., Simons, K., and Zerial, M. (1992). The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70, 715-728. - PubMed
-
- Burd, C.G., and Emr, S.D. (1998). Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell 2, 157-162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

