Fusion between phagosomes, early and late endosomes: a role for actin in fusion between late, but not early endocytic organelles
- PMID: 14617814
- PMCID: PMC307552
- DOI: 10.1091/mbc.e03-05-0334
Fusion between phagosomes, early and late endosomes: a role for actin in fusion between late, but not early endocytic organelles
Abstract
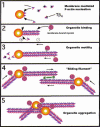
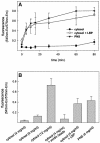
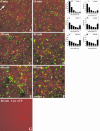
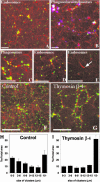
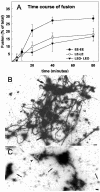
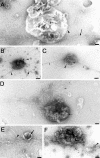
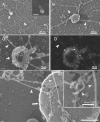
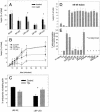
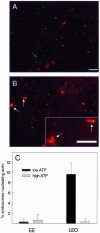
Actin is implicated in membrane fusion, but the precise mechanisms remain unclear. We showed earlier that membrane organelles catalyze the de novo assembly of F-actin that then facilitates the fusion between latex bead phagosomes and a mixture of early and late endocytic organelles. Here, we correlated the polymerization and organization of F-actin with phagosome and endocytic organelle fusion processes in vitro by using biochemistry and light and electron microscopy. When membrane organelles and cytosol were incubated at 37 degrees C with ATP, cytosolic actin polymerized rapidly and became organized into bundles and networks adjacent to membrane organelles. By 30-min incubation, a gel-like state was formed with little further polymerization of actin thereafter. Also during this time, the bulk of in vitro fusion events occurred between phagosomes/endocytic organelles. The fusion between latex bead phagosomes and late endocytic organelles, or between late endocytic organelles themselves was facilitated by actin, but we failed to detect any effect of perturbing F-actin polymerization on early endosome fusion. Consistent with this, late endosomes, like phagosomes, could nucleate F-actin, whereas early endosomes could not. We propose that actin assembled by phagosomes or late endocytic organelles can provide tracks for fusion-partner organelles to move vectorially toward them, via membrane-bound myosins, to facilitate fusion.
Figures
Similar articles
-
Disruption of the actin filament network affects delivery of endocytic contents marker to phagosomes with early endosome characteristics: the case of phagosomes with pathogenic mycobacteria.Eur J Cell Biol. 2000 Oct;79(10):735-49. doi: 10.1078/0171-9335-00092. Eur J Cell Biol. 2000. PMID: 11089922
-
ATP-dependent membrane assembly of F-actin facilitates membrane fusion.Mol Biol Cell. 2001 Jan;12(1):155-70. doi: 10.1091/mbc.12.1.155. Mol Biol Cell. 2001. PMID: 11160830 Free PMC article.
-
Ezrin promotes actin assembly at the phagosome membrane and regulates phago-lysosomal fusion.Traffic. 2011 Apr;12(4):421-37. doi: 10.1111/j.1600-0854.2011.01158.x. Epub 2011 Feb 8. Traffic. 2011. PMID: 21210911
-
Role of microtubules and myosins in Fc gamma receptor-mediated phagocytosis.Front Biosci. 2006 May 1;11:1479-90. doi: 10.2741/1897. Front Biosci. 2006. PMID: 16368530 Review.
-
Roles of the cytoskeleton and motor proteins in endocytic sorting.Adv Drug Deliv Rev. 2003 Nov 14;55(11):1385-403. doi: 10.1016/j.addr.2003.07.008. Adv Drug Deliv Rev. 2003. PMID: 14597137 Review.
Cited by
-
Annexin A8 regulates late endosome organization and function.Mol Biol Cell. 2008 Dec;19(12):5267-78. doi: 10.1091/mbc.e08-04-0383. Epub 2008 Oct 15. Mol Biol Cell. 2008. PMID: 18923148 Free PMC article.
-
Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages.Biology (Basel). 2021 Jun 22;10(7):567. doi: 10.3390/biology10070567. Biology (Basel). 2021. PMID: 34206480 Free PMC article. Review.
-
Actin-binding protein regulation by microRNAs as a novel microbial strategy to modulate phagocytosis by host cells: the case of N-Wasp and miR-142-3p.Front Cell Infect Microbiol. 2013 Jun 5;3:19. doi: 10.3389/fcimb.2013.00019. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23760605 Free PMC article.
-
Actin polymerization in the endosomal pathway, but not on the Coxiella-containing vacuole, is essential for pathogen growth.PLoS Pathog. 2018 Apr 18;14(4):e1007005. doi: 10.1371/journal.ppat.1007005. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29668757 Free PMC article.
-
Actin and phosphoinositide recruitment to fully formed Candida albicans phagosomes in mouse macrophages.J Innate Immun. 2009;1(3):244-53. doi: 10.1159/000173694. Epub 2008 Nov 12. J Innate Immun. 2009. PMID: 20375582 Free PMC article.
References
-
- Anes, E., Kuhnel, M.P., Bos, E., Pereira, J.M., Habermann, A., and Griffiths, G. (2003). Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat. Cell Biol. 5, 793-802. - PubMed
-
- Amann, K.J., and Pollard, T.D. (2000). Cellular regulation of actin network assembly. Curr. Biol. 10, R728-730. - PubMed
-
- Bader, M.F., Holz, R.W., Kumakura, K., and Vitale, N. (2002). Exocytosis: the chromaffin cell as a model system. Ann. N.Y. Acad. Sci. 971, 178-183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

