Homologous recombination in human telomerase-positive and ALT cells occurs with the same frequency
- PMID: 14618159
- PMCID: PMC1326419
- DOI: 10.1038/sj.embor.7400027
Homologous recombination in human telomerase-positive and ALT cells occurs with the same frequency
Abstract
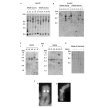
Homologous recombination is thought to be the molecular mechanism for maintaining telomere length in alternative lengthening of telomeres (ALT) cells. We used a recombination reporter system to show that the frequency of homologous recombination is the same for ALT- and telomerase-positive cells, suggesting that if ALT cells have a recombination defect it specifically involves telomeric sequences. We compared internal and telomere-adjacent positions of our reporter construct to investigate if telomeric sequences near an induced double-strand break alter the frequency of recombination between nontelomeric sequences, and found no differences among the different cell lines analysed. Our results indicate that the underlying defect in homologous recombination in ALT cells does not affect sequences independent of their chromosomal location but is likely to be primarily a specific telomeric defect.
Figures
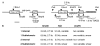

References
-
- Baird D.M. & Royle N.J. ( 1997) Sequences from higher primates orthologous to the human Xp/Yp telomere junction region reveal gross rearrangements and high levels of divergence. Hum. Mol. Genet., 6, 2291–2299. - PubMed
-
- Baur J.A., Zou Y., Shay J.W. & Wright W.E. ( 2001) Telomere position effect in human cells. Science, 292, 2075–2077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

