Effects of microinjected small GTPases on the actin cytoskeleton of human neutrophils
- PMID: 14620378
- PMCID: PMC1571180
- DOI: 10.1046/j.1469-7580.2003.00222.x
Effects of microinjected small GTPases on the actin cytoskeleton of human neutrophils
Abstract
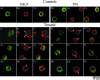
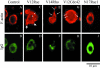
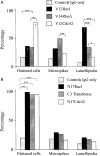
This paper describes a method for microinjection of proteins (Rho GTPases) into neutrophils and observations on the responses of the cells to these injections. Neutrophils are extremely difficult to inject because of their small size, complex morphology and fragility. To allow microinjections they must be cultured on a substrate that enables them to settle, adhere and spread. We determined that fibronectin- and/or collagen-coated coverslips are the best substrates and we used very fine needles and short microinjection times to minimize cell damage. These methods permitted us to inject up to 100 cells in a single preparation over a period of 30 min. Effects of microinjection were assessed by using tetramethylrhodamine isothiocyanate (TRITC)-phalloidin to label F-actin filaments, and observation by fluorescence and confocal scanning microscopy. Microinjection alone resulted in cell rounding and some changes in the F-actin cytoskeleton but injected cells remained adherent at the substrate, were able to respond to microinjected GTPases (V12Rac, V14RhoA, V12Cdc42) and continued to be responsive to activation by exposure to fMet-Leu-Phe (fMLP) or O-tetradecanoylphorbal 13-acetate (TPA). V12Rac caused an increase in neutrophil membrane ruffling and short protrusions from the cell membrane, whereas V14RhoA induced a large increase in punctate F-actin structures. V12Cdc42 produced focal condensation of F-actin and induced the formation of small microspikes. The differences between these responses of neutrophils and those of other similarly treated cell types are discussed. Our findings demonstrate that microinjection is a valuable technique for studying the role of individual proteins in neutrophils.
Figures


References
-
- Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 1997;110:707–720. 10.1046/j.1469-7580.2003.00222.x. - DOI - PubMed
-
- Allen WE, Zicha D, Ridley AJ, Jones GE. A role for role for Cdc42 in macrophage chemotaxis. J. Cell Biol. 1998;141:1147–1157. 10.1046/j.1469-7580.2003.00222.x. - DOI - PMC - PubMed
-
- Aspenstrom P. The Rho GTPases have multiple effects on the actin cytoskeleton. Exp. Cell Res. 1999;246:20–25. 10.1046/j.1469-7580.2003.00222.x. - DOI - PubMed
-
- Bishop AL, Hall A. The Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255. 10.1046/j.1469-7580.2003.00222.x. - DOI - PMC - PubMed
-
- Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–1149. 10.1046/j.1469-7580.2003.00222.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

