Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions
- PMID: 14622188
- PMCID: PMC1971243
- DOI: 10.1046/j.l460-9568.2003.02973.x
Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions
Abstract
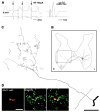
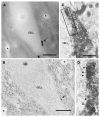
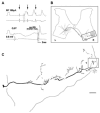
Axonal projections and neurotransmitters used by commissural interneurons mediating crossed actions of reticulospinal neurons were investigated in adult cats. Eighteen interneurons, located in or close to lamina VIII in midlumbar segments, that were monosynaptically excited by reticulospinal tract fibres and projected to contralateral motor nuclei were labelled by intracellular injection of tetramethylrhodamine-dextran and Neurobiotin. The nine most completely labelled interneurons were analysed with combined confocal and light microscopy. None of the stem axons gave off ipsilateral axon collaterals. Seven cells had axon collaterals that arborized in the contralateral grey matter in the ventral horn of the same segments. Transmitters were identified by using antibodies raised against vesicular glutamate transporters 1 and 2, glutamic acid decarboxylase and the glycine transporter 2. The axons of two cells were immunoreactive for the glycine transporter 2 and hence were glycinergic. Three cells were immunoreactive for the vesicular glutamate transporter 2 and hence were glutamatergic. None of the axons displayed immunoreactivity for glutamic acid decarboxylase. Electron microscopy of two cells revealed direct synaptic connections with motoneurons and other neurons. Axonal swellings of one neuron formed synapses with profiles in motor nuclei whereas those of the other formed synapses with other structures, including cell bodies in lamina VII. The results show that this population of commissural interneurons includes both excitatory and inhibitory cells that may excite or inhibit contralateral motoneurons directly. They may also influence the activity of motoneurons indirectly by acting through interneurons located outside motor nuclei in the contralateral grey matter but are unlikely to have direct actions on interneurons in the ipsilateral grey matter.
Figures
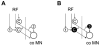





References
-
- Bannatyne BA, Maxwell DJ, Edgley SE, Hammar I, Jankowska E. Commissural interneurons in cat spinal motor pathways: identification of excitatory and inhibitory cells. J Physiol (Lond) 2003;5489:116.
-
- Bras H, Cavallari P, Jankowska E. Demonstration of initial axon collaterals of cells of origin of the ventral spinocerebellar tract in the cat. J Comp Neurol. 1988;273:584–592. - PubMed
-
- Bras H, Cavallari P, Jankowska E, Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol. 1989;290:1–15. - PubMed
-
- Cajal S, Ramon y. Histologie du Systeme Nerveux de L’homme et des Vertebres. Institute Cajal; Madrid: 1909.
-
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I Movements evoked by microstimulation. J Neurophysiol. 1990a;64:767–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

