Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast
- PMID: 14622595
- PMCID: PMC4493758
- DOI: 10.1016/s0092-8674(03)00886-9
Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast
Abstract
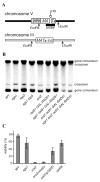
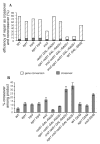
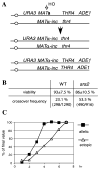
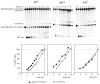
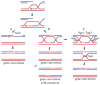
Very few gene conversions in mitotic cells are associated with crossovers, suggesting that these events are regulated. This may be important for the maintenance of genetic stability. We have analyzed the relationship between homologous recombination and crossing-over in haploid budding yeast and identified factors involved in the regulation of crossover outcomes. Gene conversions unaccompanied by a crossover appear 30 min before conversions accompanied by exchange, indicating that there are two different repair mechanisms in mitotic cells. Crossovers are rare (5%), but deleting the BLM/WRN homolog, SGS1, or the SRS2 helicase increases crossovers 2- to 3-fold. Overexpressing SRS2 nearly eliminates crossovers, whereas overexpression of RAD51 in srs2Delta cells almost completely eliminates the noncrossover recombination pathway. We suggest Sgs1 and its associated topoisomerase Top3 remove double Holliday junction intermediates from a crossover-producing repair pathway, thereby reducing crossovers. Srs2 promotes the noncrossover synthesis-dependent strand-annealing (SDSA) pathway, apparently by regulating Rad51 binding during strand exchange.
Figures

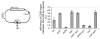
References
-
- Adams MD, McVey M, Sekelsky JJ. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science. 2003;299:265–267. - PubMed
-
- Ajima J, Umezu K, Maki H. Elevated incidence of loss of heterozygosity (LOH) in an sgs1 mutant of Saccharomyces cerevisiae: roles of yeast RecQ helicase in suppression of aneuploidy, interchromosomal rearrangement, and the simultaneous incidence of both events during mitotic growth. Mutat Res. 2002;504:157–172. - PubMed
-
- Allers T, Lichten M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell. 2001;106:47–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

