An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors
- PMID: 14623770
- PMCID: PMC1574161
- DOI: 10.1038/sj.bjp.0705577
An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors
Abstract
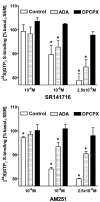
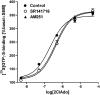
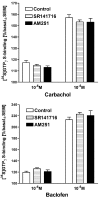
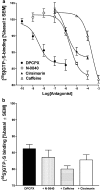
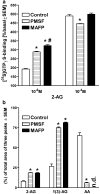
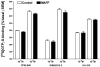
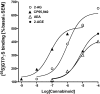
At nanomolar concentrations, SR141716 and AM251 act as specific and selective antagonists of the cannabinoid CB1 receptor. In the micromolar range, these compounds were shown to inhibit basal G-protein activity, and this is often interpreted to implicate constitutive activity of the CB1 receptors in native tissue. We show here, using [35S]GTPgammaS binding techniques, that micromolar concentrations of SR141716 and AM251 inhibit basal G-protein activity in rat cerebellar membranes, but only in conditions where tonic adenosine A1 receptor signaling is not eliminated. Unlike lipophilic A1 receptor antagonists (potency order DPCPX>>N-0840 approximately cirsimarin>caffeine), adenosine deaminase (ADA) was not fully capable in eliminating basal A1 receptor-dependent G-protein activity. Importantly, all antagonists reduced basal signal to the same extent (20%), and the response evoked by the inverse agonist DPCPX was not reversed by the neutral antagonist N-0840. These data indicate that rat brain A1 receptors are not constitutively active, but that an ADA-resistant adenosine pool is responsible for tonic A1 receptor activity in brain membranes. SR141716 and AM251, at concentrations fully effective in reversing CB1-mediated responses (10-6 m), did not reduce basal G-protein activity, indicating that CB1 receptors are not constitutively active in these preparations.4 At higher concentrations (1-2.5 x 10-5 m), both antagonists reduced basal G-protein activity in control and ADA-treated membranes, but had no effect when A1 receptor signaling was blocked with DPCPX. Moreover, the CB1 antagonists right-shifted A1 agonist dose-response curves without affecting maximal responses, suggesting competitive mode of antagonist action. The CB1 antagonists did not affect muscarinic acetylcholine or GABAB receptor signaling. When further optimizing G-protein activation assay for the labile endocannabinoid 2-arachidonoylglycerol (2-AG), we show, by using HPLC, that pretreatment of cerebellar membranes with methyl arachidonoyl fluorophosphonate (MAFP) fully prevented enzymatic degradation of 2-AG and concomitantly enhanced the potency of 2-AG. In contrast to previous claims, MAFP exhibited no antagonist activity at the CB1 receptor.6 The findings establish an optimized method with improved signal-to-noise ratio to assess endocannabinoid-dependent G-protein activity in brain membranes, under assay conditions where basal adenosinergic tone and enzymatic degradation of 2-AG are fully eliminated.
Figures
Similar articles
-
Detection of cannabinoid CB1, adenosine A1, muscarinic acetylcholine, and GABA(B) receptor-dependent G protein activity in transducin-deactivated membranes and autoradiography sections of rat retina.Cell Mol Neurobiol. 2004 Apr;24(2):243-56. doi: 10.1023/b:cemn.0000018619.18631.53. Cell Mol Neurobiol. 2004. PMID: 15176438 Free PMC article.
-
Effects of Delta9-tetrahydrocannabivarin on [35S]GTPgammaS binding in mouse brain cerebellum and piriform cortex membranes.Br J Pharmacol. 2008 Jul;154(6):1349-58. doi: 10.1038/bjp.2008.190. Epub 2008 May 19. Br J Pharmacol. 2008. PMID: 18493244 Free PMC article.
-
Selective detection of adenosine A1 receptor-dependent G-protein activity in basal and stimulated conditions of rat brain [35S]guanosine 5'-(gamma-thio)triphosphate autoradiography.Neuroscience. 1999;90(4):1265-79. doi: 10.1016/s0306-4522(98)00571-5. Neuroscience. 1999. PMID: 10338296
-
Inverse agonism and neutral antagonism at cannabinoid CB1 receptors.Life Sci. 2005 Feb 4;76(12):1307-24. doi: 10.1016/j.lfs.2004.10.025. Epub 2004 Dec 8. Life Sci. 2005. PMID: 15670612 Review.
-
Second generation CB1 receptor blockers and other inhibitors of peripheral endocannabinoid overactivity and the rationale of their use against metabolic disorders.Expert Opin Investig Drugs. 2012 Sep;21(9):1309-22. doi: 10.1517/13543784.2012.704019. Epub 2012 Jul 11. Expert Opin Investig Drugs. 2012. PMID: 22780328 Review.
Cited by
-
Endocannabinoid Receptors in the CNS: Potential Drug Targets for the Prevention and Treatment of Neurologic and Psychiatric Disorders.Curr Neuropharmacol. 2020;18(8):769-787. doi: 10.2174/1570159X18666200217140255. Curr Neuropharmacol. 2020. PMID: 32065105 Free PMC article. Review.
-
Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review.Br J Pharmacol. 2015 Feb;172(3):737-53. doi: 10.1111/bph.12944. Br J Pharmacol. 2015. PMID: 25257544 Free PMC article.
-
Role of endocannabinoids and cannabinoid-1 receptors in cerebrocortical blood flow regulation.PLoS One. 2013;8(1):e53390. doi: 10.1371/journal.pone.0053390. Epub 2013 Jan 4. PLoS One. 2013. PMID: 23308211 Free PMC article.
-
Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation.Learn Mem. 2007 Jan 3;14(1-2):63-74. doi: 10.1101/lm.439007. Print 2007 Jan-Feb. Learn Mem. 2007. PMID: 17202425 Free PMC article.
-
CB1 receptor antagonism in capuchin monkeys alters social interaction and aversive memory extinction.Psychopharmacology (Berl). 2019 Dec;236(12):3413-3419. doi: 10.1007/s00213-019-05305-0. Epub 2019 Jun 27. Psychopharmacology (Berl). 2019. PMID: 31250073
References
-
- BASS C.E., GRIFFIN G., GRIER M., MAHADEVAN A., RAZDAN R.K., MARTIN B.R. SR-141716A-induced stimulation of locomotor activity A structure–activity relationship study. Pharmacol. Biochem. Behav. 2002;74:31–40. - PubMed
-
- BISOGNO T., MELCK D., DE PETROCELLIS L., BOBROV M.Y., GRETSKAYA N.M., BEZUGLOV V.V., SITACHITTA N., GERWICK W.H., DI MARZO V. Arachidonoylserotonin and other novel inhibitors of fatty acid amide hydrolase. Biochem. Biophys. Res. Commun. 1998;248:515–522. - PubMed
-
- BREIVOGEL C.S., GRIFFIN G., DI MARZO V., MARTIN B.R. Evidence for a new G-protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. - PubMed
-
- BREIVOGEL C.S., SELLEY D.S., CHILDERS S.R. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPγS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J. Biol. Chem. 1998;273:16865–16873. - PubMed
-
- DEUTSCH D.G., OMEIR R., ARREAZA G., SALEHANI D., PRESTWICH G.D., HUANG Z., HOWLETT A. Methyl arachidonyl fluorophosphonate: a potent irreversible inhibitor of anandamide amidase. Biochem. Pharmacol. 1997;53:255–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

