Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons
- PMID: 14623865
- PMCID: PMC2173668
- DOI: 10.1083/jcb.200304128
Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons
Abstract
Spinal muscular atrophy (SMA), a common autosomal recessive form of motoneuron disease in infants and young adults, is caused by mutations in the survival motoneuron 1 (SMN1) gene. The corresponding gene product is part of a multiprotein complex involved in the assembly of spliceosomal small nuclear ribonucleoprotein complexes. It is still not understood why reduced levels of the ubiquitously expressed SMN protein specifically cause motoneuron degeneration. Here, we show that motoneurons isolated from an SMA mouse model exhibit normal survival, but reduced axon growth. Overexpression of Smn or its binding partner, heterogeneous nuclear ribonucleoprotein (hnRNP) R, promotes neurite growth in differentiating PC12 cells. Reduced axon growth in Smn-deficient motoneurons correlates with reduced beta-actin protein and mRNA staining in distal axons and growth cones. We also show that hnRNP R associates with the 3' UTR of beta-actin mRNA. Together, these data suggest that a complex of Smn with its binding partner hnRNP R interacts with beta-actin mRNA and translocates to axons and growth cones of motoneurons.
Figures
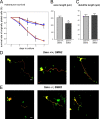
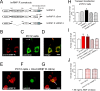

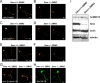
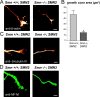

References
-
- Bechade, C., P. Rostaing, C. Cisterni, R. Kalisch, V. La Bella, B. Pettmann, and A. Triller. 1999. Subcellular distribution of survival motor neuron (SMN) protein: possible involvement in nucleocytoplasmic and dendritic transport. Eur. J. Neurosci. 11:293–304. - PubMed
-
- Blanc, V., N. Navaratnam, J.O. Henderson, S. Anant, S. Kennedy, A. Jarmuz, J. Scott, and N.O. Davidson. 2001. Identification of GRY-RBP as an apolipoprotein B RNA-binding protein that interacts with both apobec-1 and apobec-1 complementation factor to modulate C to U editing. J. Biol. Chem. 276:10272–10283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

