Spatio-temporal propagation of Ca2+ signals by cyclic ADP-ribose in 3T3 cells stimulated via purinergic P2Y receptors
- PMID: 14623867
- PMCID: PMC2173669
- DOI: 10.1083/jcb.200307016
Spatio-temporal propagation of Ca2+ signals by cyclic ADP-ribose in 3T3 cells stimulated via purinergic P2Y receptors
Abstract
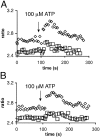
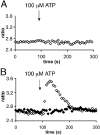
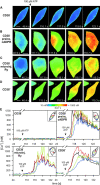
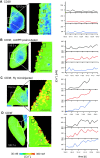
The role of cyclic ADP-ribose in the amplification of subcellular and global Ca2+ signaling upon stimulation of P2Y purinergic receptors was studied in 3T3 fibroblasts. Either (1) 3T3 fibroblasts (CD38- cells), (2) 3T3 fibroblasts preloaded by incubation with extracellular cyclic ADP-ribose (cADPR), (3) 3T3 fibroblasts microinjected with ryanodine, or (4) 3T3 fibroblasts transfected to express the ADP-ribosyl cyclase CD38 (CD38+ cells) were used. Both preincubation with cADPR and CD38 expression resulted in comparable intracellular amounts of cyclic ADP-ribose (42.3 +/- 5.2 and 50.5 +/- 8.0 pmol/mg protein). P2Y receptor stimulation of CD38- cells yielded a small increase of intracellular Ca2+ concentration and a much higher Ca2+ signal in CD38-transfected cells, in cADPR-preloaded cells, or in cells microinjected with ryanodine. Confocal Ca2+ imaging revealed that stimulation of ryanodine receptors by cADPR or ryanodine amplified localized pacemaker Ca2+ signals with properties resembling Ca2+ quarks and triggered the propagation of such localized signals from the plasma membrane toward the internal environment, thereby initiating a global Ca2+ wave.
Figures

Similar articles
-
Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system.Ann N Y Acad Sci. 2004 Dec;1028:176-91. doi: 10.1196/annals.1322.021. Ann N Y Acad Sci. 2004. PMID: 15650244 Review.
-
Activation of CD38 by interleukin-8 signaling regulates intracellular Ca2+ level and motility of lymphokine-activated killer cells.J Biol Chem. 2005 Jan 28;280(4):2888-95. doi: 10.1074/jbc.M409592200. Epub 2004 Nov 19. J Biol Chem. 2005. PMID: 15556942
-
The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells.Endocrinology. 2004 Feb;145(2):881-9. doi: 10.1210/en.2003-0774. Epub 2003 Oct 16. Endocrinology. 2004. PMID: 14563702
-
cADP-ribose/ryanodine channel/Ca2+-release signal transduction pathway in mesangial cells.Am J Physiol Renal Physiol. 2001 Jul;281(1):F91-F102. doi: 10.1152/ajprenal.2001.281.1.F91. Am J Physiol Renal Physiol. 2001. PMID: 11399650
-
["The CD38-cyclic ADP-ribose signal system": molecular mechanism and biological significance].Nihon Yakurigaku Zasshi. 1999 Sep;114(3):131-9. doi: 10.1254/fpj.114.131. Nihon Yakurigaku Zasshi. 1999. PMID: 10553576 Review. Japanese.
Cited by
-
Paracrine ADP Ribosyl Cyclase-Mediated Regulation of Biological Processes.Cells. 2022 Aug 24;11(17):2637. doi: 10.3390/cells11172637. Cells. 2022. PMID: 36078044 Free PMC article. Review.
-
8-Bromo-cyclic inosine diphosphoribose: towards a selective cyclic ADP-ribose agonist.Biochem J. 2009 Jul 29;422(1):139-49. doi: 10.1042/BJ20082308. Biochem J. 2009. PMID: 19492987 Free PMC article.
-
The Complex Interplay between Toxic Hallmark Proteins, Calmodulin-Binding Proteins, Ion Channels, and Receptors Involved in Calcium Dyshomeostasis in Neurodegeneration.Biomolecules. 2024 Jan 31;14(2):173. doi: 10.3390/biom14020173. Biomolecules. 2024. PMID: 38397410 Free PMC article. Review.
-
The role of Ca2+ signaling in Parkinson's disease.Dis Model Mech. 2017 May 1;10(5):519-535. doi: 10.1242/dmm.028738. Dis Model Mech. 2017. PMID: 28468938 Free PMC article. Review.
-
Extracellular NAD+ regulates intracellular calcium levels and induces activation of human granulocytes.Biochem J. 2006 Feb 1;393(Pt 3):697-704. doi: 10.1042/BJ20051302. Biochem J. 2006. PMID: 16225456 Free PMC article.
References
-
- Albrieux, M., H.C. Lee, and M. Villaz. 1998. Ca2+ signalling by cyclic ADP-ribose, NAADP, and inositol trisphosphate are involved in distinct functions in ascidian oocytes. J. Biol. Chem. 273:14566–14574. - PubMed
-
- Atherton, T.J., S. Wilson, R.I. Morrey, and A. Waterfall. 1997. Digital Confocal Imaging Research Report RR333. Department of Computer Science, University of Warwick, Coventry, UK. 15 pp.
-
- Berridge, M.J., P. Lipp, and M.D. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21. - PubMed
-
- Bootman, M.D., M.J. Berridge, and P. Lipp. 1997. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 91:367–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

