Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen
- PMID: 14623912
- PMCID: PMC1592647
- DOI: 10.1084/jem.20031051
Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen
Abstract
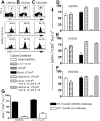
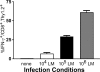
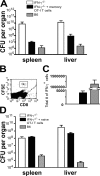
Interferon (IFN)-gamma plays an important role in the innate immune response against intracellular bacterial pathogens. It is commonly thought that natural killer cells are the primary source of this cytokine that is involved in activating antibacterial effects in infected cells and polarizing CD4+ T cells toward the Th1 subset. However, here we show that both effector and memory CD8+ T cells have the potential to secrete IFN-gamma in response to interleukin (IL)-12 and IL-18 in the absence of cognate antigen. We demonstrate that memory CD8+ T cells specific for the ovalbumin protein secrete IFN-gamma rapidly after infection with wild-type Listeria monocytogenes (LM). Furthermore, small numbers of ovalbumin-specific, memory CD8+ T cells can reduce spleen and liver bacterial counts in IFN-gamma-deficient mice 3 d after LM infection. Up-regulation of the receptors for IL-12 and IL-18 provides a mechanism for the ability of memory CD8+ T cells to respond in this antigen nonspecific manner. Thus, CD8+ T cells play an important role in the innate immune response against intracellular pathogens by rapidly secreting IFN-gamma in response to IL-12 and IL-18.
Figures




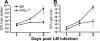
References
-
- Portnoy, D.A. 1992. Innate immunity to a facultative intracellular bacterial pathogen. Curr. Opin. Immunol. 4:20–24. - PubMed
-
- Unanue, E.R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11–25. - PubMed
-
- Mombaerts, P., J. Arnoldi, F. Russ, S. Tonegawa, and S.H. Kaufmann. 1993. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 365:53–56. - PubMed
-
- MacMicking, J.D., C. Nathan, G. Hom, N. Chartrain, D.S. Fletcher, M. Trumbauer, K. Stevens, Q.W. Xie, K. Sokol, N. Hutchinson, et al. 1995. Altered responses to bacterial-infection and endotoxic-shock in mice lacking inducible nitric-oxide synthase. Cell. 81:641–650. - PubMed
-
- Beckerman, K.P., H.W. Rogers, J.A. Corbett, R.D. Schreiber, M.L. McDaniel, and E.R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J. Immunol. 150:888–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

